当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Concise and Versatile Synthesis of Alkaloids from Kopsia tenuis: Total Synthesis of (±)‐Lundurine A and B
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2014-05-25 , DOI: 10.1002/anie.201400464 Shigeru Arai , Masaya Nakajima , Atsushi Nishida
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2014-05-25 , DOI: 10.1002/anie.201400464 Shigeru Arai , Masaya Nakajima , Atsushi Nishida
|
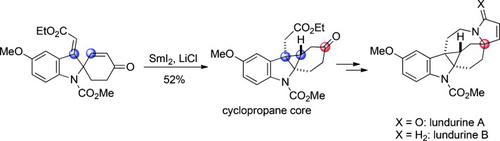
A total synthesis of (±)‐lundurines A and B is described. These natural products have a unique hexacyclic skeleton which includes a cyclopropane‐fused indoline. A stereospecific construction of the pentasubstituted cyclopropane core was achieved, by radical cyclization using SmI2, with perfect stereoselectivity. Cyclizations to give seven‐ and five‐membered heterocycles, under palladium and ruthenium catalysis, respectively, accomplished the total syntheses. The late‐stage construction of the F ring by ring‐closing metathesis enabled access to the title compounds from a spiroindoline intermediate which is a common structure of other kopsia alkaloids.
中文翻译:

香菜中生物碱的简便,多功能合成:(±)-Lundurine A和B的全合成
描述了(±)-鸟尿苷A和B的总合成。这些天然产物具有独特的六环骨架,其中包括环丙烷稠合的二氢吲哚。通过使用SmI 2进行自由基环化,以完美的立体选择性实现了五取代环丙烷核的立体有择结构。在钯和钌的催化下,分别生成七元和五元杂环的环化反应完成了全部合成过程。F环通过闭环易位的后期构建使得能够从螺吲哚啉中间体获得标题化合物,螺中间体是其他kopsia生物碱的常见结构。
更新日期:2014-05-25
中文翻译:

香菜中生物碱的简便,多功能合成:(±)-Lundurine A和B的全合成
描述了(±)-鸟尿苷A和B的总合成。这些天然产物具有独特的六环骨架,其中包括环丙烷稠合的二氢吲哚。通过使用SmI 2进行自由基环化,以完美的立体选择性实现了五取代环丙烷核的立体有择结构。在钯和钌的催化下,分别生成七元和五元杂环的环化反应完成了全部合成过程。F环通过闭环易位的后期构建使得能够从螺吲哚啉中间体获得标题化合物,螺中间体是其他kopsia生物碱的常见结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号