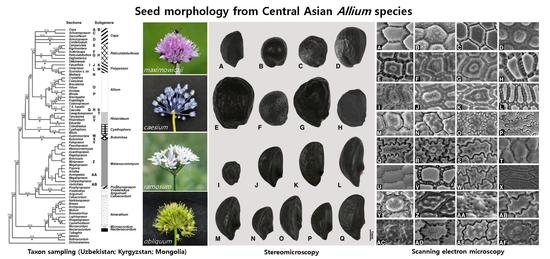
Seed Morphology of Allium L. (Amaryllidaceae) from Central Asian Countries and Its Taxonomic Implications
Abstract
:1. Introduction
2. Results
2.1. Seed Macro-Morphology
2.2. Seed Testa Sculptures
2.2.1. Subgenus Allium
2.2.2. Subgenus Butomissa (Salisb) N. Friesen
2.2.3. Subgenus Cepa (Mill.) Radić
2.2.4. Subgenus Melanocrommyum (Webb et Berth.) Rouy
2.2.5. Subgenus Polyprason Radić
2.2.6. Subgenus Reticulatobulbosa (Kamelin) N. Friesen
2.2.7. Subgenus Rhizirideum (G.Don. ex Koch) Wendelbo s.s.
3. Discussion
3.1. Subgenus Allium
3.2. Subgenus Butomissa (Salisb.) N. Friesen
3.3. Subgenus Cepa (Mill.) Radić
3.4. Subgenus Melanocrommyum (Webb et Berth.) Rouy
3.5. Subgenus Polyprason Radić
3.6. Subgenus Reticulatobulbosa (Kamelin) N. Friesen
3.7. Subgenus Rhizirideum (G. Don ex Koch) Wendelbo s.s.
4. Materials and Methods
4.1. Taxon Sampling
4.2. Light Microscopic Analysis
4.3. Scanning Electron Microscopic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Material
References
- Herden, T.; Hanelt, P.; Friesen, N. Phylogeny of Allium L. subgenus Anguinum (G. Don. ex W.D.J. Koch) N. Friesen (Amaryllidaceae). Mol. Phylogen. Evol. 2016, 95, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Friesen, N.; Fritsch, R.M.; Blattner, F.R. Phylogeny and new intrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA its sequences. Aliso 2006, 22, 372–395. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhou, S.D.; He, X.J.; Yu, Y.; Zhang, Y.C.; Wei, X.Q. Phylogeny and biogeography of Allium (Amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to China. Ann. Bot. 2010, 106, 709–773. [Google Scholar] [CrossRef] [PubMed]
- Gurushidze, M.; Mashayekhi, S.; Blattner, F.R.; Friesen, N.; Fritsch, R.M. Phylogenetic relationships of wild and cultivated species of Allium section Cepa inferred by nuclear rDNA ITS sequence analysis. Plant Syst. Evol. 2007, 269, 259–269. [Google Scholar] [CrossRef]
- Gurushidze, M.; Fritsch, R.M.; Blattner, F.R. Phylogenetic analysis of Allium subg. Melanocrommyum infers cryptic species and demands a new sectional classification. Mol. Phylogen. Evol. 2008, 49, 997–1007. [Google Scholar] [CrossRef]
- Gurushidze, M.; Fritsch, R.M.; Blattner, F.R. Species level phylogeny of Allium subgenus Melanocrommyum: Incomplete lineage sorting, hybridization and trnF gene duplication. Taxon 2010, 59, 829–840. [Google Scholar] [CrossRef]
- Fritsch, R.M.; Abbasi, M. A Taxonomic Review of Allium Subg. Melanocrommyum in Iran; IPK Gatersleben: Gatersleben, Germany, 2013; ISBN 9783981309638. [Google Scholar]
- Wheeler, E.J.; Mashayekhi, S.; Mcneal, D.W.; Columbus, J.T.; Pires, J.C. Molecular systematics of Allium subgenus Amerallium (Amaryllidaceae) in North America. Am. J. Bot. 2013, 100, 701–711. [Google Scholar] [CrossRef]
- Mashayeckhi, S.; Columbus, J.T. Evolution of leaf blade anatomy in Allium (Amaryllidaceae) subgenus Amerallium with a focus on the North American species. Am. J. Bot. 2014, 100, 63–85. [Google Scholar] [CrossRef] [Green Version]
- Li, M.J.; Tan, J.B.; Xie, D.F.; Huang, D.Q.; Gao, Y.D.; He, X.J. Revisiting the evolutionary events in Allium subgenus Cyathophora (Amaryllidaceae): Insights into the effect of the Hengduan Mountains Region (HMR) uplift and Quaternary climatic fluctuations to the environmental changes in the Qinghai-Tibet Plateau. Mol. Phylogen. Evol. 2016, 94, 802–813. [Google Scholar] [CrossRef]
- Seregin, A.P.; Anaćkov, G.; Friesen, N. Molecular and morphological revision of the Allium saxatile group (Amaryllidaceae): Geographical isolation as the driving force of underestimated speciation. Bot. J. Linn. Soc. 2015, 178, 67–101. [Google Scholar] [CrossRef] [Green Version]
- Sinitsyna, T.A.; Herden, T.; Friesen, N. Dated phylogeny and biogeography of the Eurasian Allium section Rhizirideum (Amaryllidaceae). Plant Syst. Evol. 2016, 302, 1311–1328. [Google Scholar] [CrossRef]
- Friesen, N.; Smirnov, S.V.; Shmakov, A.I.; Herden, T.; Oyuntsetseg, B.; Hurka, H. Allium species of section Rhizomatosa, early members of the Cenrtal Aisan steppe vegetation. Flora 2020, 263, 1–13. [Google Scholar] [CrossRef]
- Bothmer, R.V. Studies in the Aegean Flora. XXI. Biosystematic studies in the Allium ampeloprasum complex. Opera Bot. 1974, 34, 1–107. [Google Scholar]
- De Wilde-Duyfjes, B.E.E. A revision of the genus Allium, L. (Liliaceae) in Africa. Meded. Landbouwhogesch. Wagening. 1976, 76-11, 1–237. [Google Scholar]
- Pastor, J. Contribuciòn al estudio de las semillas de las especies de Allium de la Peninsula Ibèricae islas Baleares. Lagascalia 1981, 10, 207–216. [Google Scholar]
- Kruse, J. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. Die Kult. 1984, 32, 89–101. [Google Scholar] [CrossRef]
- Kruse, J. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. II. Die Kult. 1986, 34, 207–228. [Google Scholar] [CrossRef]
- Kruse, J. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. III. Die Kult. 1988, 36, 355–368. [Google Scholar] [CrossRef]
- Kruse, J. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. IV. Feddes Repert. 1994, 105, 457–471. [Google Scholar] [CrossRef]
- Cesmedziev, I.; Terzijski, D. A scanning electron microscopic study of the spermoderm in Allium subg. Codonoprasum (Alliaceae). Bocconea 1997, 5, 755–758. [Google Scholar]
- Ilarslan, H.; Koyuncu, M. Türkiye’de yetisen bazi endemic Allium (soğan) türlerinin tohum morfolojileri. Ot. Sist. Bot. Derg. 1997, 4, 99–116. [Google Scholar]
- Fritsch, R.M.; Kruse, J.; Adler, K.; Rutten, T. Testa sculptures in Allium L. subg. Melanocrommyum (Webb and Berth.) Rouy (Alliaceae). Feddes Reper. 2006, 117, 250–263. [Google Scholar] [CrossRef]
- Neshati, F.; Fritsch, R.M. Seed characters and testa sculptures of some Iranian Allium L. species (Alliaceae). Feddes Reper. 2009, 120, 322–332. [Google Scholar] [CrossRef]
- Choi, H.J.; Cota-Sanchez, J.H. A taxonomic revision of Allium (Alliaceae) in the Canadian prairie provinces. Botany 2010, 88, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Oh, B.U. A partial revision of Allium (Amaryllidaceae) in Korea and northeastern China. Bot. J. Linn. Soc. 2011, 167, 153–211. [Google Scholar] [CrossRef] [Green Version]
- Bednorz, L.; Krzymińska, A.; Czarna, A. Seed morphology and testa sculptures of some Allium L. species (Alliaceae). Acta Agrobot. 2011, 64, 33–38. [Google Scholar] [CrossRef]
- Celep, F.; Koyuncu, M.; Fritsch, R.M.; Kahraman, A.; Doğan, M. Taxonomic importance of seed morphology in Allium (Amaryllidaceae). Syst. Bot. 2012, 37, 893–912. [Google Scholar] [CrossRef]
- Duman, H.; Ekşi, G.; Özbek, F. Two new species Allium L. sect Allium (Amaryllidaceae) from Turkey. Plant Syst. Evol. 2017, 303, 1271–1291. [Google Scholar] [CrossRef]
- Choi, H.J.; Giussani, L.M.; Jang, C.G.; Oh, B.U.; Cota-Sanchez, J.H. Systematics of disjunct northeastern Asian and northern North American Allium (Amaryllidaceae). Botany 2012, 90, 491–508. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tan, D.Y. Seed testa micromorphology of thirty-eight species of Allium (Amaryllidaceae) from central Asia, and its taxonomic implications. Nord. J. Bot. 2017, 35, 189–200. [Google Scholar] [CrossRef]
- Veiskarami, G.; Khodayari, H.; Heubl, G.; Zarre, S. Seed surface ultrastructure as an efficient tool for species delimitation in the Allium ampeloprasum L. alliance (Amaryllidaceae, Allioideae). Microsc. Res. Tech. 2018, 81, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Deniz, İ.G.; Genç, İ.; Sari, D. Morphological and molecular data reveal a new species of Allium (Amaryllidaceae) from SW Anatolia, Turkey. Phytotaxa 2015, 212, 283–292. [Google Scholar] [CrossRef]
- Rola, K. Cell pattern and ultrasculpture of bulb tunic of selected Allium species (Amaryllidaceae) and their diagnostic value. Acta Biol. Cracov. Bot. 2014, 56, 28–41. [Google Scholar] [CrossRef]
- Khorasani, M.; Saedi Mehrvarz, S.h.; Zarre, S. Bulb tunic anatomy and its taxonomic implication in Allium L. (Amaryllidaceae: Allioideae). Plant Biosyst. Inter. J. Deal. All Asp. Plant Biol. 2018, 152, 1311–1328. [Google Scholar] [CrossRef]
- Khorasani, M.; Saedi Mehrvarz, S.; Zarre, S. Scape anatomy and its systematic implication in Allium stipitatum species complex (Amaryllidaceae). Nord. J. Bot. 2018, 152, 1311–1328. [Google Scholar] [CrossRef]
- Friesen, N. The genus Allium L. in the flora of Mongolia. Feddes Reper. 1995, 106, 59–81. [Google Scholar] [CrossRef]
- Lazkov, G.A.; Sultanova, B.A. Checklist of vascular plants of Kyrgyzstan. Norrlinia 2011, 24, 1–166. [Google Scholar]
- Sennikov, A.N.; Lazkov, G.A. Allium formosum Sennikov and Lazkov (Amaryllidaceae), a new species from Kyrgyzstan. Phytokeys 2013, 21, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Oyuntsetseg, B.; Darikhand, N.; Friesen, N. Allium carolinianum DC. A new species to the Outer Mongolia. Turczaninowia 2013, 16, 88–90. [Google Scholar]
- Urgamal, M.; Oyuntsetseg, B.; Nyambayar, D.; Dulamsuren, C. Conspectus of the Vascular Plants of Mongolia; Admon Printing: Ulaanbaatar, Mongolia, 2014; ISBN 9789997303561. [Google Scholar]
- Sennikov, A.N.; Tojibaev, K.S.; Khassanov, F.O.; Beshko, N.Y. The flora of Uzbekistan project. Phytotaxa 2016, 282, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Filimonova, Z.N. Morfologija semjan sredneaziatskikh vidovr. Allium L. Introd. I Akklim. Rast Na Ukr. 1971, 8, 111–116. [Google Scholar]
- Brullo, S.; Pavone, P.; Salmeri, C. Allium aetnense (Amaryllidaceae), a new species from Sicily. Plant Biosyst. 2013, 147, 835–843. [Google Scholar] [CrossRef]
- Deniz, İ.G.; Genç, İ.; Ince, A.G.; Aykurt, C.; Elmasulu, S.; Sümbül, H.; Sönmez, S.; Citak, S. Taxonomic data supporting differences between Allium elmaliense and Allium cyrilli. Biologia 2013, 68, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Genç, İ.; Ozhatay, F.N. Allium efeae (Amaryllidaceae), a new species from northwest Anatolia, Turkey. Turk. J. Bot. 2014, 38, 1022–1025. [Google Scholar] [CrossRef]
- Özhatay, N.; Koçyigit, M.; Brullo, S.; Salmeri, C. Allium istanbulense, a new autumnal species of A sect Codonoprasum (Amaryllidaceae) from Turkey and its taxonomic position among allied species. Phytotaxa 2018, 334, 152–166. [Google Scholar] [CrossRef]
- Yildirim, H. Allium sultanae-ismailii (Amaryllidaceae), a new species from eastern Turkey. Phytotaxa 2019, 403, 039–046. [Google Scholar] [CrossRef]
- Khassanov, F.O. Taxonomical and Ethnobotanical Aspects of Allium Species from Middle Asia with Particular Reference to Subgenus Allium. In The Allium Genomes; Shigyo, M., Khar, A., Abdelrahman, M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2018; pp. 11–21. ISBN 9783319958248. [Google Scholar] [CrossRef]
- Barthlott, W. Epidermal and seed surface characters of plants: Systematic applicability and some evolutionary aspects. Nord. J. Bot. 1981, 1, 345–355. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baasanmunkh, S.; Lee, J.K.; Jang, J.E.; Park, M.S.; Friesen, N.; Chung, S.; Choi, H.J. Seed Morphology of Allium L. (Amaryllidaceae) from Central Asian Countries and Its Taxonomic Implications. Plants 2020, 9, 1239. https://doi.org/10.3390/plants9091239
Baasanmunkh S, Lee JK, Jang JE, Park MS, Friesen N, Chung S, Choi HJ. Seed Morphology of Allium L. (Amaryllidaceae) from Central Asian Countries and Its Taxonomic Implications. Plants. 2020; 9(9):1239. https://doi.org/10.3390/plants9091239
Chicago/Turabian StyleBaasanmunkh, Shukherdorj, Jae Kyoung Lee, Ju Eun Jang, Min Su Park, Nikolai Friesen, Sungwook Chung, and Hyeok Jae Choi. 2020. "Seed Morphology of Allium L. (Amaryllidaceae) from Central Asian Countries and Its Taxonomic Implications" Plants 9, no. 9: 1239. https://doi.org/10.3390/plants9091239