Phytochemical Characterization, Antioxidant Activity, and Cytotoxicity of Methanolic Leaf Extract of Chlorophytum Comosum (Green Type) (Thunb.) Jacq
Abstract
:1. Introduction
2. Results
2.1. GC-MS Analysis
2.2. Phytochemical Screening of Fractions
2.3. Antioxidant Activity of Fractions
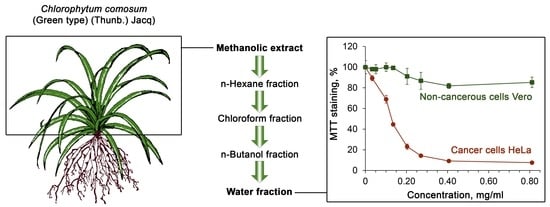
2.4. Cytotoxicity
2.5. MALDI-TOF Mass Spectrometry of Water Fraction of Methanolic Extract of Leaves of C. comosum
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Sample Preparation
4.4. Extraction and Fractionation
4.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Methanolic Leaf Extract
4.6. Matrix-Assisted Laser Desorption/Ionization (MALDI) Time-of-Flight (TOF) Mass Spectrometry
4.7. Determination of Total Phenolics
4.8. Determination of Total Tannins
4.9. Determination of Total Flavonoids
4.10. Determination of Chlorophyll a, Chlorophyll b, and Total Carotenoid
4.11. Determination of Carbohydrates
4.12. Ferric Reducing Power Assay
4.13. Assessment of Total Antioxidant Activity (TAA)
4.14. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
4.15. Cytotoxicity Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Kaushik, N. Saponins of Chlorophytum species. Phytochem. Rev. 2005, 4, 191–196. [Google Scholar] [CrossRef]
- Patil, S.M.; Chandanshive, V.V.; Tamboli, A.S.; Adsul, A.A.; Yadav, S.R.; Govindwar, S.P. Analysis of genetic variability in endemic medicinal plants of genus Chlorophytum from the Indian subcontinent using amplified fragment length polymorphism marker. Comptes Rendus Biol. 2015, 338, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Munyao, J.N.; Dong, X.; Yang, J.-X.; Mbandi, E.M.; Wanga, V.O.; Oulo, M.A.; Saina, J.K.; Musili, P.M.; Hu, G.-W. Complete Chloroplast Genomes of Chlorophytum comosum and Chlorophytum gallabatense: Genome Structures, Comparative and Phylogenetic Analysis. Plants 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deore, S.L.; Jajoo, N.B.; Chittam, K.P.; Deshmukh, T.A. Comparative Pharmacognostic, Phytochemical and Biological evaluation between five Chlorophytum species. Pharmacogn. J. 2015, 7, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Khare, C.P. Indian Medicinal Plants. In An Illustrated Dictionary; Khare, C.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 68. [Google Scholar] [CrossRef]
- Yang, X.; Chen, A.; Ma, Y.; Gao, Y.; Gao, Z.; Fu, B.; Sun, F.; Qiao, J.; Li, Q.; Wan, S.; et al. Encyclopedic Reference of Traditional Chinese Medicine; Springer: Berlin/Heidelberg, Germany, 2007; p. 567. [Google Scholar] [CrossRef]
- Fan, C.; Jin, H.; Wu, L.; Zhang, Y.; Ye, R.D.; Zhang, W.; Zhang, Y. An Exploration of Traditional Chinese Medicinal Plants with Anti-Inflammatory Activities. Evid. Based. Complement. Alternat. Med. 2017, 2017, 1231820. [Google Scholar] [CrossRef] [PubMed]
- Tabuti, J.R.; Lye, K.A.; Dhillion, S.S. Traditional herbal drugs of Bulamogi, Uganda: Plants, use and administration. J. Ethnopharmacol. 2003, 88, 19–44. [Google Scholar] [CrossRef]
- Vijaya, N.K.; Chavan, P.D. Chlorophytum borivilianum (Safed musli): A Review. Pharmacogn. Rev. 2009, 3, 154–169. [Google Scholar]
- Chauhan, R.; Quraishi, A.; Jadhav, S.K.; Keshavkant, S. A comprehensive review on pharmacological properties and biotechnological aspects of Genus Chlorophytum. Acta Physiol. Plant. 2016, 38, 116. [Google Scholar] [CrossRef]
- Thakur, M.; Bhargava, S.; Dixit, V.K. Immunomodulatory Activity of Chlorophytum borivilianum Sant. F. Evid. Based Complement. Alternat. Med. 2007, 4, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.S.; Sen, S.S.; Chi, C.; Kim, H.J.; Yun, S.; Park, S.C.; Sukumaran, V. Chlorophytum borivilianum Polysaccharide Fraction Provokes the Immune Function and Disease Resistance of Labeo rohita against Aeromonas hydrophila. J. Immunol. Res. 2015, 2015, 256510. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Kaur, S. Protective efficacy of Chlorophytum borivilianum root extract against murine visceral leishmaniasis by immunomodulating the host responses. J. Ayurveda Integr. Med. 2020, 11, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Connellan, P.; Deseo, M.A.; Morris, C.; Dixit, V.K. Immunomodulatory Polysaccharide from Chlorophytum borivilianum Roots. Evid. Based Complement. Alternat. Med. 2011, 2011, 598521. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.S.; Bhutani, K.K. Effects of Chlorophytum arundinaceum, Asparagus adscendens and Asparagus racemosus on pro-inflammatory cytokine and corticosterone levels produced by stress. Phytother. Res. 2010, 24, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Lande, A.A.; Ambavade, S.D.; Swami, U.S.; Adkar, P.P.; Ambavade, P.D.; Waghamare, A.B. Saponins isolated from roots of Chlorophytum borivilianum reduce acute and chronic inflammation and histone deacetylase. J. Integr. Med. 2015, 13, 25–33. [Google Scholar] [CrossRef]
- O’Donnell, G.; Bucar, F.; Gibbons, S. Phytochemistry and antimycobacterial activity of Chlorophytum inornatum. Phytochemistry 2006, 67, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.; Nazifi, A.B.; Odoma, S.; Shehu, S.; Danjuma, N.M. Antinociceptive activity of methanol extract of Chlorophytum alismifolium tubers in murine model of pain: Possible involvement of α2-adrenergic receptor and KATP channels. J. Tradit. Complement. Med. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Soni, B.; Dalwadi, N.; Madamwar, D. Chlorophytum borivilianum as potential terminator of free radicals in various in vitro oxidation systems. Drug. Chem. Toxicol. 2010, 33, 173–182. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, M. Antioxidant and modulatory role of Chlorophytum borivilianum against arsenic induced testicular impairment. J. Environ. Sci. 2012, 24, 2159–2165. [Google Scholar] [CrossRef]
- Narasimhan, S.; Govindarajan, R.; Vijayakumar, M.; Mehrotra, S. Free radical scavenging potential of Chlorophytum tuberosum Baker. J. Ethnopharmacol. 2006, 104, 423–425. [Google Scholar] [CrossRef]
- Ashraf, M.F.; Abd Aziz, M.; Stanslas, J.; Ismail, I.; Abdul Kadir, M. Assessment of antioxidant and cytotoxicity activities of saponin and crude extracts of Chlorophytum borivilianum. Sci. World J. 2013, 2013, 216894. [Google Scholar] [CrossRef]
- Kenjale, R.D.; Shah, R.K.; Sathaye, S.S. Anti-stress and anti-oxidant effects of roots of Chlorophytum borivilianum (Santa Pau & Fernandes). Indian J. Exp. Biol. 2007, 45, 974–979. [Google Scholar] [PubMed]
- Narasimhan, S.; Govindarajan, R.; Madhavan, V.; Thakur, M.; Dixit, V.K.; Mehrotra, S.; Madhusudanan, K.P. Action of (2-->1)Fructo-oligopolysaccharide fraction of Chlorophytum borivilianum against Streptozotocin-Induced oxidative stress. Planta Med. 2006, 72, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Meena, P.; Verma, S.; Kumar, M.; Kumar, A. Anti-tumour, anti-mutagenic and chemomodulatory potential of Chlorophytum borivilianum. Asian Pac. J. Cancer Prev. 2010, 11, 327–334. [Google Scholar] [PubMed]
- Shinde, S.S.; Patil, S.M.; Rane, N.R.; Adsul, A.A.; Gholve, A.R.; Pawar, P.K.; Yadav, S.R.; Govindwar, S.P. Comprehensive investigation of free radical quenching potential, total phenol, flavonoid and saponin content, and chemical profiles of twelve Chlorophytum Ker Gawl species. Indian J. Nat. Prod. Resour. 2016, 7, 125–134. [Google Scholar]
- Thakur, M.; Thompson, D.; Connellan, P.; Deseo, M.A.; Morris, C.; Dixit, V.K. Improvement of penile erection, sperm count and seminal fructose levels in vivo and nitric oxide release in vitro by ayurvedic herbs. Andrologia 2011, 43, 273–277. [Google Scholar] [CrossRef]
- Mahajan, G.K.; Mahajan, A.Y.; Mahajan, R.T. Efficacy of aphrodisiac plants towards improvement in semen quality and motility in infertile males. J. Complement. Integr. Med. 2012, 9, 6. [Google Scholar] [CrossRef]
- Rath, S.K.; Panja, A.K. Clinical evaluation of root tubers of Shweta Musali (Chlorophytum borivilianum L.) and its effect on semen and testosterone. Ayu 2013, 34, 273–275. [Google Scholar] [CrossRef] [Green Version]
- Kenjale, R.; Shah, R.; Sathaye, S. Effects of Chlorophytum borivilianum on sexual behaviour and sperm count in male rats. Phytother. Res. 2008, 22, 796–801. [Google Scholar] [CrossRef]
- Ray, S.; Chatterjee, K.; De, D.; Ghosh, D. Bioefficacy of hydromethanolic extract of tuber of Chlorophytum borivilianum (Safed Musli) for the management of male infertility in cyproterone acetate-treated albino rats. Andrologia 2014, 46, 659–671. [Google Scholar] [CrossRef]
- Thakur, M.; Chauhan, N.S.; Bhargava, S.; Dixit, V.K. A comparative study on aphrodisiac activity of some ayurvedic herbs in male albino rats. Arch. Sex. Behav. 2009, 38, 1009–1015. [Google Scholar] [CrossRef]
- Thakur, M.; Bhargava, S.; Praznik, W.; Loeppert, R.; Dixit, V.K. Effect of Chlorophytum borivilianum Santapau and Fernandes on sexual dysfunction in hyperglycemic male rats. Chin. J. Integr. Med. 2009, 15, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singhal, S.; Kumar, N.; Rao, C.M.; Sumalatha, S.; Dave, J.; Dave, R.; Nandakumar, K. Standardised extract of Safed Musli (Chlorophytum borivilianum) increases aphrodisiac potential besides being safe in male Wistar rats. Andrologia 2016, 48, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, N.; Kumar, K.E.; Rekha, S.S.; Muniandy, S.; Salleh, N. Chlorophytum borivilianum (Safed Musli) root extract prevents impairment in characteristics and elevation of oxidative stress in sperm of streptozotocin-induced adult male diabetic Wistar rats. BMC Complementary Altern. Med. 2014, 14, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giribabu, N.; Kumar, K.E.; Rekha, S.S.; Muniandy, S.; Salleh, N. Chlorophytum borivilianum root extract maintains near normal blood glucose, insulin and lipid profile levels and prevents oxidative stress in the pancreas of streptozotocin-induced adult male diabetic rats. Int. J. Med. Sci. 2014, 11, 1172–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi, V.; Kumar, R.; Pandey, K.; Joshi, B.S.; Roy, R.; Madhusudanan, K.P.; Tiwari, P.; Srivastava, A.K. Structure and activities of a steroidal saponin from Chlorophytum nimonii (Grah) Dalz. Nat. Prod. Res. 2009, 23, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Narasimhacharya, A.V. Ameliorative effect of Chlorophytum borivilianum root on lipid metabolism in hyperlipaemic rats. Clin. Exp. Pharmacol. Physiol. 2007, 34, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Areshidze, D.A.; Timchenko, L.D.; Gulyukin, M.I.; Kozlova, M.A.; Rzhepakovsky, I.V.; Syomin, I.A.; Andreyuk, V.A. Hepatoprotective effect of preparations produced from Chlorophytum comosum (L.) at experimental toxic damage in wistar rats. Pharmacologyonline 2016, 2, 81–90. [Google Scholar]
- Areshidze, D.; Timchenko, L.; Kozlova, M. The use of enzymatic hydrolyzate of Chlorophytum comosum with experimental toxic liver damage in rats. Am. J. Biomed. Sci. Res. 2013, 1, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, H.; Kuwabara, H.; Ishikawa, S.; Mochizuki, M. Apoptosis Induced in Human Cell Lines by a Butanol Extract from Chlorophytum comosum Roots. J. Health Sci. 2005, 51, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Tandon, M.; Shukla, Y.N. A bibenzyl xyloside from Chlorophytum arundinaceum. Phytochemistry 1993, 32, 1624–1625. [Google Scholar] [CrossRef]
- Deore, S.L.; Khadabadi, S.S. Isolation and characterization of phytoconstituents from Chlorophytum borivilianum. Pharmacogn. Res. 2010, 2, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Patil, V.N.; Deokule, S.S. Pharmacognostic study of Chlorophytum tuberosum Baker. Int. J. Ayurveda Res. 2010, 1, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, M.; Shukla, Y.N.; Thakur, R.S. 4-hydroxy-8,11-oxidoheneicosanol and other constituents from Chlorophytum arundinaceum roots. Phytochemistry 1992, 31, 2525–2526. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kanmoto, T.; Sashida, Y.; Nishino, A.; Satomi, Y.; Nishino, H. Steroidal saponins from the underground parts of Chlorophytum comosum and their inhibitory activity on tumour promoter-induced phospholipids metabolism of HeLa cells. Phytochemistry 1996, 41, 1405–1410. [Google Scholar] [CrossRef]
- Rizvi, M.Z.; Kukreja, A.K.; Bisht, N.S. In vitro propagation of an endangered medicinal herb Chlorophytum borivilianum Sant. et Fernand. through somatic embryogenesis. Physiol. Mol. Biol. Plants 2010, 16, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, S.X.; Li, X.C.; Xiong, Y.; Dong, Y.; Chai, H.; Fransworth, N.R.; Pezzuto, J.M.; Fong, H.H. Isolation and characterization of cytotoxic saponin chloromaloside A from Chlorophytum malayense. Planta Med. 2000, 66, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Mitaine-Offer, A.C.; Kaushik, N.; Miyamoto, T.; Paululat, T.; Mirjolet, J.F.; Duchamp, O.; Lacaille-Dubois, M.A. Cytotoxic spirostane-type saponins from the roots of Chlorophytum borivilianum. J. Nat. Prod. 2009, 72, 177–181. [Google Scholar] [CrossRef]
- Sharma, R.; Thakur, G.S.; Sanodiya, B.S.; Pandey, M.; Bisen, P.S. Saponin: A Wonder Drug from Chlorophytum Species. Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 503–515. [Google Scholar]
- Tabopda, T.K.; Mitaine-Offer, A.C.; Paululat, T.; Delemasure, S.; Dutartre, P.; Ngadjui, B.T.; Lacaille-Dubois, M.A. Steroidal saponins from Chlorophytum deistelianum. Phytochemistry 2016, 126, 34–40. [Google Scholar] [CrossRef]
- Acharya, D.; Mitaine-Offer, A.C.; Kaushik, N.; Miyamoto, T.; Paululat, T.; Mirjolet, J.F.; Duchamp, O.; Lacaille-Dubois, M.A. Steroidal saponins from Chlorophytum orchidastrum. J. Nat. Prod. 2010, 73, 7–11. [Google Scholar] [CrossRef]
- Kumar, S.; Kalra, S.; Kumar, S.; Kaur, J.; Singh, K. Differentially expressed transcripts from leaf and root tissue of Chlorophytum borivilianum: A plant with high medicinal value. Gene 2012, 511, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chakraborthy, G.S. Antioxidant Activity of the Successive Extracts of Chlorophytum borivilianum Leaves. Asian J. Chem. 2008, 20, 5782–5784. [Google Scholar]
- Joshi, A.; Chauhan, R.S. Cytotoxicity studies of Chlorophytum borivilianum against BHK-21 cells. J. Biol. Chem. Res. 2013, 30, 302–309. [Google Scholar]
- Dill, V.; Pfaff, F.; Zimmer, A.; Beer, M.; Eschbaumer, M. Adherent and suspension baby hamster kidney cells have a different cytoskeleton and surface receptor repertoire. PLoS ONE 2021, 16, e0246610. [Google Scholar] [CrossRef]
- Adhami, S.; Farooqi, H.; Abdin, M.Z.; Prasad, R.; Malik, A.A. Chemical Profiling of Chlorophytum comosum (Thunb.) Jaques by GCMS/LC-ESI-MS and its Antiproliferative Effects on Human Carcinoma Cell Lines. Anticancer Agents Med. Chem. 2020, 21, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Bajpai, P.; Siddiqui, M.H.; Sayyed, U.; Tiwari, R.; Shekh, R.; Mishra, K.; Kapoor, V.K. Elucidation of the Chemopreventive Role of Stigmasterol Against Jab1 in Gall Bladder Carcinoma. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Poda, G.I. Application of ALOGPS 2.1 to predict log D distribution coefficient for Pfizer proprietary compounds. J. Med. Chem. 2004, 47, 5601–5604. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic acid: Between doubts and certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Van Rooijen, M.A.; Mensink, R.P. Palmitic Acid Versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review. Nutrients 2020, 12, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, H.; Ntambi, J.M. The fate and intermediary metabolism of stearic acid. Lipids 2005, 40, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tester, R.F. Lactose, Maltose, and Sucrose in Health and Disease. Mol. Nutr. Food Res. 2020, 64, e1901082. [Google Scholar] [CrossRef] [PubMed]
- Kluch, M.; Socha-Banasiak, A.; Pacześ, K.; Borkowska, M.; Czkwianianc, E. The role of disaccharidases in the digestion—Diagnosis and significance of their deficiency in children and adults. Pol Merkur Lek. 2020, 49, 275–278. [Google Scholar]
- McCall, J.M.; DeCristofaro, C.; Elliott, L. Oral sucrose for pain control in nonneonate infants during minor painful procedures. J. Am. Assoc. Nurse Pract. 2013, 25, 244–252. [Google Scholar] [CrossRef]
- Puertolas, M.V.; Fifi, A.C. The Role of Disaccharidase Deficiencies in Functional Abdominal Pain Disorders-A Narrative Review. Nutrients 2018, 10, 1835. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef]
- Balamurugan, R.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 667, 410–418. [Google Scholar] [CrossRef]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J. BUON 2018, 23, 1420–1425. [Google Scholar]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Dixit, S.; Ali, D.; Alqahtani, S.M.; Alkahtani, S.; Alarifi, S. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Devel. Ther. 2015, 9, 2793–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Yang, C.S. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Batta, A.K.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 2006, 55, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.F.; Hooper, S.N.; Ismail, H.A. Antihypercholesterolemic studies with sterols: Beta-sitosterol and stigmasterol. J. Pharm. Sci. 1979, 68, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Andriamiarina, R.; Laraki, L.; Pelletier, X.; Debry, G. Effects of stigmasterol-supplemented diets on fecal neutral sterols and bile acid excretion in rats. Ann. Nutr. Metab. 1989, 33, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Xie, Z.; Lu, Q.; Luo, S. Stigmasterol protects against Ang II-induced proliferation of the A7r5 aortic smooth muscle cell-line. Food Funct. 2015, 6, 2266–2272. [Google Scholar] [CrossRef]
- Lifsey, H.C.; Kaur, R.; Thompson, B.H.; Bennett, L.; Temel, R.E.; Graf, G.A. Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine. J. Nutr. Biochem. 2020, 76, 108263. [Google Scholar] [CrossRef]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009, 80, 123–126. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.; Zhao, P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.C.; Park, J.H.; Budesinsky, M.; Kasal, A.; Han, Y.H.; Koo, B.S.; Lee, S.I.; Lee, D.U. Antimutagenic constituents from the thorns of Gleditsia sinensis. Chem. Pharm. Bull. 2005, 53, 561–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminu, R.; Umar, I.A.; Rahman, M.A.; Ibrahim, M.A. Stigmasterol retards the proliferation and pathological features of Trypanosoma congolense infection in rats and inhibits trypanosomal sialidase in vitro and in silico. Biomed. Pharmacother. 2017, 89, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.I.B.; Oliveira, S.M.; Tonello, R.; Rossato, M.F.; da Silva Brum, E.; Ferreira, J.; Trevisan, G. Anti-nociceptive effect of stigmasterol in mouse models of acute and chronic pain. Naunyn Schmiedeberg Arch. Pharmacol. 2017, 390, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Yu, C.; Hu, P.F.; Bao, J.P.; Tang, J.L.; Wu, L.D. Stigmasterol blocks cartilage degradation in rabbit model of osteoarthritis. Acta Biochim. Pol. 2012, 59, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Antwi, A.O.; Obiri, D.D.; Osafo, N.; Essel, L.B.; Forkuo, A.D.; Atobiga, C. Stigmasterol Alleviates Cutaneous Allergic Responses in Rodents. Biomed. Res. Int. 2018, 2018, 3984068. [Google Scholar] [CrossRef] [Green Version]
- Antwi, A.O.; Obiri, D.D.; Osafo, N. Stigmasterol Modulates Allergic Airway Inflammation in Guinea Pig Model of Ovalbumin-Induced Asthma. Mediat. Inflamm. 2017, 2017, 2953930. [Google Scholar] [CrossRef]
- Antwi, A.O.; Obiri, D.D.; Osafo, N.; Forkuo, A.D.; Essel, L.B. Stigmasterol inhibits lipopolysaccharide-induced innate immune responses in murine models. Int. Immunopharmacol. 2017, 53, 105–113. [Google Scholar] [CrossRef]
- Ahmad Khan, M.; Sarwar, A.H.M.G.; Rahat, R.; Ahmed, R.S.; Umar, S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int. Immunopharmacol. 2020, 85, 106642. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Liu, J.; Pan, X.; Zhao, Q. Stigmasterol Exerts Neuro-Protective Effect Against Ischemic/Reperfusion Injury Through Reduction Of Oxidative Stress And Inactivation Of Autophagy. Neuropsychiatr. Dis. Treat. 2019, 15, 2991–3001. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Yang, J.; He, J.; Chen, X.; Zhang, H.; Jia, M.; Liu, K.; Jia, C.; Pan, Y.; Wei, J. Stigmasterol alleviates cerebral ischemia/reperfusion injury by attenuating inflammation and improving antioxidant defenses in rats. Biosci Rep. 2020, 40, BSR20192133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adebiyi, O.E.; Olopade, J.O.; Olayemi, F.O. Sodium metavanadate induced cognitive decline, behavioral impairments, oxidative stress and down regulation of myelin basic protein in mice hippocampus: Ameliorative roles of β-spinasterol, and stigmasterol. Brain Behav. 2018, 8, e01014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.N.; Moon, I.S. Stigmasterol promotes neuronal migration via reelin signaling in neurosphere migration assays. Nutr. Neurosci. 2020, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Bhuiyan, M.M.H.; Moon, I.S. Stigmasterol activates Cdc42-Arp2 and Erk1/2-Creb pathways to enrich glutamatergic synapses in cultures of brain neurons. Nutr. Res. 2018, 56, 71–78. [Google Scholar] [CrossRef]
- Haque, M.N.; Moon, I.S. Stigmasterol upregulates immediate early genes and promotes neuronal cytoarchitecture in primary hippocampal neurons as revealed by transcriptome analysis. Phytomedicine 2018, 46, 164–175. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Jung, J.M.; Kim, J.M.; Cai, M.; Liu, X.; Hong, J.G.; Lee, C.H.; Lee, K.R.; Ryu, J.H. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur. J. Pharmacol. 2012, 676, 64–70. [Google Scholar] [CrossRef]
- Tao, C.; Shkumatov, A.A.; Alexander, S.T.; Ason, B.L.; Zhou, M. Stigmasterol accumulation causes cardiac injury and promotes mortality. Commun. Biol. 2019, 2, 20. [Google Scholar] [CrossRef]
- Islam, M.T.; de Alencar, M.V.; da Conceição Machado, K.; da Conceição Machado, K.; de Carvalho Melo-Cavalcante, A.A.; de Sousa, D.P.; de Freitas, R.M. Phytol in a pharma-medico-stance. Chem. Biol. Interact. 2015, 240, 60–73. [Google Scholar] [CrossRef]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on phytol. Food Chem. Toxicol. 2010, 48, S59–S63. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bin-Jumah, M.; Othman, S.I.; Khafaga, A.F.; Shaheen, H.M.; Samak, D.; Shehata, A.M.; Allam, A.A.; Abd El-Hack, M.E. The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review. Molecules 2020, 25, 1941. [Google Scholar] [CrossRef] [Green Version]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, A.; Barros, L.; Ferreira, I.C. A comparison of the phenolic profile and antioxidant activity of different Cichorium spinosum L. ecotypes. J. Sci. Food Agric. 2018, 98, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartler, S.M. Apparent Hela cell contamination of human heteroploid cell lines. Nature 1968, 217, 750–751. [Google Scholar] [CrossRef]
- Lavappa, K.S. Survey of ATCC stocks of human cell lines for HeLa contamination. In Vitro 1978, 14, 469–475. [Google Scholar] [CrossRef]
- Smith, K.R.; Hayat, F.; Andrews, J.F.; Migaud, M.E.; Gassman, N.R. Dihydroxyacetone Exposure Alters NAD(P)H and Induces Mitochondrial Stress and Autophagy in HEK293T Cells. Chem. Res. Toxicol. 2019, 32, 1722–1731. [Google Scholar] [CrossRef]
- Smith, K.R.; Granberry, M.; Tan, M.C.B.; Daniel, C.L.; Gassman, N.R. Dihydroxyacetone induces G2/M arrest and apoptotic cell death in A375P melanoma cells. Environ. Toxicol. 2018, 33, 333–342. [Google Scholar] [CrossRef]
- Petersen, A.B.; Wulf, H.C.; Gniadecki, R.; Gajkowska, B. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat. Res. 2004, 560, 173–186. [Google Scholar] [CrossRef]
- Perer, J.; Jandova, J.; Fimbres, J.; Jennings, E.Q.; Galligan, J.J.; Hua, A.; Wondrak, G.T. The sunless tanning agent dihydroxyacetone induces stress response gene expression and signaling in cultured human keratinocytes and reconstructed epidermis. Redox Biol. 2020, 36, 101594. [Google Scholar] [CrossRef]
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-Hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809. [Google Scholar] [CrossRef]
- Severin, I.; Dumont, C.; Jondeau-Cabaton, A.; Graillot, V.; Chagnon, M.C. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol. Lett. 2010, 192, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.N.; Wang, Y.R.; Zheng, C.H.; Feng, K. Biotransformation of 5-hydroxymethylfurfural into 2,5-dihydroxymethylfuran by Ganoderma sessile and toxicological assessment of both compounds. AMB Express 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Nemes, D.; Kovacs, R.; Nagy, F.; Mezo, M.; Poczok, N.; Ujhelyi, Z.; Peto, A.; Feher, P.; Fenyvesi, F.; Varadi, J.; et al. Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms-Assessment of Cytotoxicity and Antimicrobial Activity. Molecules 2018, 23, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, A.F.; Glasscock, C.; McClanahan, D.R.; Benson, J.D.; Higgins, A.Z. Toxicity Minimized Cryoprotectant Addition and Removal Procedures for Adherent Endothelial Cells. PLoS ONE 2015, 10, e0142828. [Google Scholar] [CrossRef] [Green Version]
- Hossler, P.; McDermott, S.; Racicot, C.; Chumsae, C.; Raharimampionona, H.; Zhou, Y.; Ouellette, D.; Matuck, J.; Correia, I.; Fann, J.; et al. Cell culture media supplementation of uncommonly used sugars sucrose and tagatose for the targeted shifting of protein glycosylation profiles of recombinant protein therapeutics. Biotechnol. Prog. 2014, 30, 1419–1431. [Google Scholar] [CrossRef]
- Yu, Y.C.; Yu, M.M. Mass spectrum of Heterocycloalkanes of Rhodiola Rosea. 2018. Available online: https://www.researchgate.net/publication/328447521_Mass_spectrum_of_Heterocycloalkanes_of_Rhodiola_Rosea_hongjingtian?channel=doi&linkId=5bce9a2b4585152b144ebadb&showFulltext=true (accessed on 17 December 2021).
- Clingman, A.L.; Richtmyer, N.K. Aryl Thioglycopyranosides, Aryl Glycopyranosyl Sulfones, and the Novel Oxidation-Acetylation of Aryl 1-Thio-β-D-glucopyranosides to 6-O-Acetyl-β-D-glucopyranosyl Aryl Sulfones. J. Org. Chem. 1964, 29, 1782–1787. [Google Scholar] [CrossRef]
- Coatney, G.R.; Cooper, W.C.; Eddy, N.B.; Greenbeeg, J. Survey of antimalarial agents. In Chemotherapy of Plasmodium gallinaceum Infections; Toxicity; Correlation of Structure and Action, 9th ed.; Public health monograph; Government Printing Office: Washington, DC, USA, 1953; p. 214. [Google Scholar]
- Hays, J.B.; Sussman, M.L.; Glass, T.W. Inhibition by 6-O-tosyl galactosides of beta-galactoside phosphorylation and transport by the lactose phosphotransferase system of Staphylococcus aureus. J. Biol. Chem. 1975, 250, 8834–8839. [Google Scholar] [CrossRef]
- Alsanosy, R.; Alhazmi, H.A.; Sultana, S.; Abdalla, A.N.; Ibrahim, Y.; Al Bratty, M.; Banji, D.; Khardali, I.; Khalid, A. Phytochemical Screening and Cytotoxic Properties of Ethanolic Extract of Young and Mature Khat Leaves. J. Chem. 2020, 2020, 7897435. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.S.; Lay, J.O.; Liyanage, R. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from rice bran. Peptides 2010, 31, 1629–1634. [Google Scholar] [CrossRef]
- Simmaco, M.; De Biase, D.; Severini, C.; Aita, M.; Erspamer, G.F.; Barra, D.; Bossa, F. Purification and characterization of bioactive peptides from skin extracts of Rana esculenta. Biochim. Biophys. Acta 1990, 1033, 318–323. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L.; Robert, M.C.; Juillerat, M.A. Peptides from Lactobacillus hydrolysates of bovine milk caseins inhibit prolyl-peptidases of human colon cells. J. Agric. Food Chem. 2011, 59, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P. CancerPPD: A database of anticancer peptides and proteins. Nucleic Acids Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.; Shin, S.Y.; Lee, S.; Kang, J.H.; Kim, S.D.; Ryu, P.D.; Hahm, K.S.; Kim, Y. Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A(1-8)-magainin 2(1-12) and its analogues, on their antibiotic activities and structures. Biochemistry 2000, 39, 11855–11864. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Rahman, M.A.; Uddin, M.M.N.; Rahman, M.M.; Uddin, M.Z.; Dash, R.; Layzu, C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complementary Altern. Med. 2015, 15, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Redha, A.; Siddiqui, S.A.; Ibrahim, S.A. Advanced extraction techniques for Berberis species phytochemicals: A review. Int. J. Food Sci. Technol. 2021, 56, 5485–5496. [Google Scholar] [CrossRef]
- Singh, M.; Shakya, S.; Soni, V.K.; Dangi, A.; Kumar, N.; Bhattacharya, S.M. The n-Hexane and Chloroform Fractions of Piper Betle, L. Trigger Different Arms of Immune Responses in BALB/c Mice and Exhibit Antifilarial Activity Against Human Lymphatic Filarid Brugia Malayi. Int. Immunopharmacol. 2009, 9, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Barabanov, P.V.; Gerasimov, A.V.; Blinov, A.V.; Kravtsov, A.A.; Kravtsov, V.A. Influence of nanosilver on the efficiency of pisum sativum crops germination. Ecotoxicol. Environ. Saf. 2018, 147, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V.; et al. Effect of Selenium Nanoparticles on Germination of Hordeum Vulgare Barley Seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Rajan, K. Analytical Techniques in Biochemistry and Molecular Biology; Springer: New York, NY, USA, 2011; pp. 67–77. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Khalifa, I.; Walayat, N.; Liu, J.; Muhammad Ahsan, H.; Irshad, S.; Barakat, H.; Lorenzo, J.M.; Pateiro, M.; et al. Effectof Structurally Different Pectin onDough Rheology, Structure, Pastingand Water Distribution Properties of Partially Meat-Based Sugar Snap Cookies. Foods 2021, 10, 2692. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complementary Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moualek, I.; Aiche, G.I.; Guechaoui, N.M.; Lahcene, S.; Houali, K. Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.S.V.G.; Reddy, S.G.; Babu, P.P.; Reddy, A.R. The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran. BMC Complementary Altern. Med. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piskov, S.; Timchenko, L.; Grimm, W.D.; Rzhepakovsky, I.; Avanesyan, S.; Sizonenko, M.; Kurchenko, V. Effects of various drying methods on some physico-chemical properties and the antioxidant profile and ACE inhibition activity of oyster mushrooms (Pleurotus ostreatus). Foods 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzhepakovsky, I.; Siddiqui, S.A.; Avanesyan, S.; Benlidayi, M.; Dhingra, K.; Dolgalev, A.; Enukashvily, N.; Fritsch, T.; Heinz, V.; Kochergin, S.; et al. Anti-arthritic effect of chicken embryo tissue hydrolyzate against adjuvant arthritis in rats (X-ray microtomographic and histopathological analysis). Food Sci. Nutr. 2021, 9, 5648–5669. [Google Scholar] [CrossRef] [PubMed]
- Heikal, A.; Elsebai, M.; Salama, A.; Taha, H. Comparative study between in vivo- and in vitro-derived extracts of cactus (Opuntis ficus-indica L. Mill) against prostate and mammary cancer cell lines. Heliyon 2021, 7, e08016. [Google Scholar] [CrossRef]



| Peak No. | RT | Name of the Phytoconstituents | Structure | Mol. Formula | Molecular Weight | Peak Area % | LogPo/w | Chemical Class |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.354 | 1,3-Dihydroxyacetone |  | C3H6O3 | 90 | 2.85 | −1.95 | Saccharide |
| 2 | 7.732 | Glycerol |  | C3H8O3 | 92 | 1.01 | −1.93 * | Polyol |
| 3 | 9.049 | 5-Hydroxymethylfurfural |  | C6H6O3 | 126 | 1.15 | −0.17 * | Aromatic aldehyde |
| 4 | 11.311 | Sucrose |  | C12H22O11 | 342 | 4.5 | −2.63 * | Disaccharide |
| 5 | 15.2 | 7,11,15-trimethyl-3-methylidenehexadec-1-ene (Neophytadiene) |  | C20H38 | 278 | 9.57 | 8.12 * | Isoprenoid hydrocarbon |
| 6 | 15.267 | 2-Hexadecene, 3,7,11,15-tetramethyl-, [R-[R*,R*-(E)]]- (2-Phytene) |  | C20H40 | 280 | 1.15 | 8.81 * | Isoprenoid hydrocarbon |
| 7 | 15.365 | E-6-Octadecen-1-ol acetate |  | C20H38O2 | 310 | 1.57 | 8.34 * | Fatty alcohol ester |
| 8 | 15.5 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) |  | C20H40O | 296 | 2.75 | 7.89 * | Diterpene alcohol |
| 9 | 15.876 | n-Hexadecanoic acid(Palmitic acid) |  | C16H32O2 | 256 | 7.66 | 7.17 | Fatty acid |
| 10 | 17.019 | 9,12-Octadecadienoic acid (Z,Z)- (Linoleic Acid) |  | C18H32O2 | 280 | 41.27 | 7.05 [58] | Fatty acid |
| 11 | 17.182 | Octadecanoic acid (Stearic acid) |  | C18H36O2 | 284 | 4.32 | 8.23 [58] | Fatty acid |
| 12 | 17.611 | 4′-Methylphenyl-1C-sulfonyl-β-d-galactoside |  | C13H18O7S | 318 | 2.88 | −1.21 * | Sulfoneglycoside |
| 13 | 18.17 | cis-7-Dodecen-1-yl acetate (Looplure) |  | C14H26O2 | 226 | 0.8 | 5.66 * | Fatty alcohol ester |
| 14 | 19.418 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester (2-Palmitoylglycerol) |  | C19H38O4 | 330 | 2.24 | 5.77 * | Fatty acid ester |
| 15 | 20.309 | Methyl 6-o-[1-methylpropyl]-β-d-galactopyranoside |  | C11H22O6 | 250 | 2.66 | −0.30 * | Monosaccharide |
| 16 | 20.927 | 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl) ethyl ester (2-Linoleoyl Glycerol) |  | C21H38O4 | 354 | 4.04 | 5.55 * | Fatty acid ester |
| 17 | 20.993 | Methyl (Z)-5,11,14,17-eicosatetraenoate |  | C21H34O4 | 318 | 2.05 | 6.82 * | Fatty acid ester |
| 18 | 31.348 | Stigmasterol |  | C29H48O | 412 | 3.09 | 6.95 * | Stigmastane |
| 19 | 33.008 | γ-Sitosterol |  | C29H50O | 414 | 4.44 | 7.27 * | Stigmastane |
| Bioactive Compounds | n-Hexane Fraction | Chloroform Fraction | n-Butanol Fraction | Water Fraction |
|---|---|---|---|---|
| Relative content of dry matter, % | 19.9 ± 0.5 | 22.8 ± 0.57 | 23.4 ± 0.4 | 33.9 ± 0.7 |
| Concentration of extracted matter in DMSO, mg/mL | 50 ± 2.0 * | 100 ± 2.0 | 100 ± 3.0 | 100 ± 3.0 |
| Total phenolic content (TPC), mg GAE/mL | 1.08 ± 0.02 | 5.6 ± 0.1 | 19.9 ± 0.5 | 0.51 ± 0.02 |
| Tannins, mg/mL | 1.73 ± 0.04 | 4.22 ± 0.1 | 15.75 ± 0.4 | 1.77 ± 0.03 |
| Total flavonoid content (TFC), mg QE/mL | 0.05 ± 0.001 | ND ** | 0.09 ± 0.001 | ND |
| Chlorophyll a, mg/mL | 3.16 ± 0.05 | 0.16 ± 0.004 | ND | ND |
| Chlorophyll b, mg/mL | 1.77 ± 0.02 | 0.26 ± 0.01 | ND | ND |
| Carotenoids, mg/mL | 0.74 ± 0.02 | 0.62 ± 0.02 | ND | ND |
| Reducing sugars, mg/mL | 1.53 ± 0.03 | 9.18 ± 0.1 | 22.2 ± 0.5 | 1.28 ± 0.02 |
| Antioxidant Activity Criterion | n-Hexane Fraction | Chloroform Fraction | n-Butanol Fraction | Water Fraction |
| Reducing power, mg AAE eq/mL | 0.39 ± 0.01 | 6.25 ± 0.2 | 33.1 ± 2.5 | 0.58 ± 0.07 |
| ABTS radical scavenging activity, mg TEs/mL | 0.27 ± 0.01 | 0.36 ± 0.01 | 0.81 ± 0.05 | 0.27 ± 0.02 |
| Total antioxidant activity (TAA), mg AAE eq/mL | 9.7 ± 0.32 | 17.4 ± 0.6 | 35.9 ± 4.5 | 1.8 ± 0.9 |
| Chemical Mass, Da | ID | Sequence | Activity | Int. |
|---|---|---|---|---|
| 681 | - | - | - | 730 |
| - | 8252 | EQRPR | anticancer | - |
| 1385 | - | - | - | 675 |
| - | 3194 | FLPAIAGILSQLF~ | hemolytic | - |
| - | 8311 | FFVAPFPEVFGK | anticancer | - |
| - | 9291 | KKLFKKILKKL~ | antifungal | - |
| - | 9466 | FKCRRWQWR | antibacterial | - |
| 1486 | - | - | - | 1335 |
| 2979 | KKAVRRQEAVDAL | CaMKII inhibitor | - | |
| - | 2989 | KKALRRDEAVDAL | CaMKII inhibitor | - |
| - | 2990 | KKALRRNEAVDAL | CaMKII inhibitor | - |
| - | 2991 | KKALRRQEGVDAL | CaMKII inhibitor | - |
| - | 3190 | SSSKEENRIIPGGI | antibacterial | - |
| - | 5450 | GLFDAIGNLLGGLGLG | antibacterial | - |
| - | 5474 | GLFDIVKKIAGHIA | antibacterial | - |
| - | 8335 | SDIPNPIGSENSEK | antibacterial | - |
| - | 9458 | RWQWRWQWR | antibacterial | - |
| - | 9783 | GEHGGAGMGGGQFQPV | alpha-amylase inhibitor | |
| - | 9784 | GEHGGAGMGGGQFQPV | pancreatic lipase inhibitor | |
| 9785 | GEHGGAGMGGGQFQPV | lipoxygenase inhibitor | ||
| 9786 | GEHGGAGMGGGQFQPV | cyclooxygenase-1 inhibitor | ||
| 9787 | GEHGGAGMGGGQFQPV | cyclooxygenase-2 inhibitor | ||
| 1503 | - | - | - | 1083 |
| - | 2987 | KKALRREEAVDAL | CaMKII inhibitor | - |
| - | 3006 | KKALYRQEAVDAL | CaMKII inhibitor | - |
| - | 3008 | KKALRYQEAVDAL | CaMKII inhibitor | - |
| - | 3920 | GLFDIIKKIAESIG | antibacterial | - |
| - | 5454 | GLFDIIKKIAESIG | antibacterial | - |
| - | 5457 | LDIVKKVVGAFGSLG | antibacterial | - |
| - | 9294 | WKLFKKILKWL~ | antifungal | - |
| - | 9295 | WKLFKKILKWL~ | hemolytic | - |
| - | 9313 | WKLFKKILKKLG | antifungal | - |
| - | 9314 | WKLFKKILKKLG | hemolytic | - |
| 2173 | - | - | - | 2403 |
| 3822 | GLLRRLRKKIGEIFKKYG | antibacterial | - | |
| 7053 | KWKLFKKIKFLHSAKKF | anticancer | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzhepakovsky, I.V.; Areshidze, D.A.; Avanesyan, S.S.; Grimm, W.D.; Filatova, N.V.; Kalinin, A.V.; Kochergin, S.G.; Kozlova, M.A.; Kurchenko, V.P.; Sizonenko, M.N.; et al. Phytochemical Characterization, Antioxidant Activity, and Cytotoxicity of Methanolic Leaf Extract of Chlorophytum Comosum (Green Type) (Thunb.) Jacq. Molecules 2022, 27, 762. https://doi.org/10.3390/molecules27030762
Rzhepakovsky IV, Areshidze DA, Avanesyan SS, Grimm WD, Filatova NV, Kalinin AV, Kochergin SG, Kozlova MA, Kurchenko VP, Sizonenko MN, et al. Phytochemical Characterization, Antioxidant Activity, and Cytotoxicity of Methanolic Leaf Extract of Chlorophytum Comosum (Green Type) (Thunb.) Jacq. Molecules. 2022; 27(3):762. https://doi.org/10.3390/molecules27030762
Chicago/Turabian StyleRzhepakovsky, Igor V., David A. Areshidze, Svetlana S. Avanesyan, Wolf D. Grimm, Natalya V. Filatova, Aleksander V. Kalinin, Stanislav G. Kochergin, Maria A. Kozlova, Vladimir P. Kurchenko, Marina N. Sizonenko, and et al. 2022. "Phytochemical Characterization, Antioxidant Activity, and Cytotoxicity of Methanolic Leaf Extract of Chlorophytum Comosum (Green Type) (Thunb.) Jacq" Molecules 27, no. 3: 762. https://doi.org/10.3390/molecules27030762