Floral Development Reveals the Existence of a Fifth Staminode on the Labellum of Basal Globbeae
- 1Department of Biological Sciences, Faculty of Science, Kanagawa University, Kanagawa, Japan
- 2Department of Biology, Tokyo Gakugei University, Tokyo, Japan
- 3College of Life Sciences and Environment, Hengyang Normal University, Hengyang, China
- 4Royal Botanic Garden Edinburgh, Edinburgh, United Kingdom
We observed the floral development of Hemiorchis burmanica and two species of Gagnepainia, which make up the sister group of Globba in Globbeae, as well as a selected number of species of Globba. Our observations revealed that in Gagnepainia, a “fifth staminode,” develops as part of the labellum, while it is generally lost in other Zingiberaceae. The fifth staminode, however, was not initiated in the labellum of Hemiorchis. A fifth staminode was also observed in Globba geoffrayi but was not found in all other species of Globba investigated. While the staminode becomes an integral part of the labellum in Gagnepainia, it remains small and almost aborts at later stages of development in G. geoffrayi. These results indicate that the development of a fifth staminode could be a recurring plesiomorphic trait for Globbeae, only retained in G. geoffrayi. The retention of the fifth staminode and its heterochronic shift may be linked with the mechanical constriction within the flower bud. The results may also support the interpretation of an atavistic expression of a once lost staminode.
Introduction
The monocots are generally characterized by a common trimerous pentacyclic floral Bauplan (Dahlgren et al., 1985). This trimerous Bauplan (i.e., P3 + 3A3 + 3G3 or K3C3A3 + 3G3) is the most remarkable floral organization of the monocots in the evolutionary context of a derivation from the basal angiosperms (Ronse De Craene, 2010; Iwamoto et al., 2018; Figure 1A). In Zingiberaceae, the flower is basically the expression of this floral Bauplan, although its flower developed several distinct features linked with monosymmetry. The flower has a sepal and petal tube consisting of three elements each, a single massive stamen and a slender style inserted between the two pollen sacs of the stamen. These characters are shared with Costaceae (Kress, 1990), considered as the sister group of Zingiberaceae in Zingiberales based on several molecular phylogenetic analyses (Kress et al., 2001; Barrett et al., 2014; Sass et al., 2016). Costaceae, however, differs from Zingiberaceae by its labellum, which is made up of five fused staminodes (Kress, 1990).
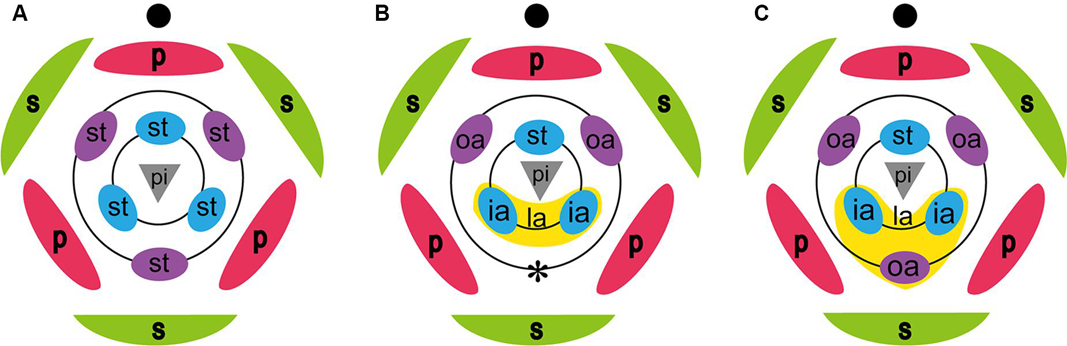
Figure 1. Floral diagrams of a generalized monocot, Zingiberaceae, and possible floral diagram of Hemiorchis and Gagnepainia. (A) Representative monocot. (B) Zingiberaceae. The labellum is composed of two inner staminodes (ia), and the abaxial outer stamen is lost (*). (C) Possible floral diagram of Hemiorchis and Gagnepainia. The labellum is composed of three staminodes, two inner staminodes, and the abaxial outer stamen (oa). ia, inner androecial element (staminode); oa, outer androecial element (staminode); pi; pistil; s, sepal; st, stamen. Black circle in each figure indicates the main axis.
The large labellum characteristic for the flower of Zingiberaceae is composed of the two lateral staminodes of the inner whorl, while two lateral staminodes of the outer whorl develop as petaloid organs and the abaxial staminode of the outer whorl, the “fifth staminode,” is considered to be lost (Kress, 1990; Kress et al., 2002; Figure 1B). Studies of floral development in several species of Zingiberaceae confirmed that the labellum develops from two inner androecial primordia (lateral staminodes), as in Alpinia (Song et al., 2007), Curcuma (Fukai and Udomdee, 2005), Globba (Box and Rudall, 2006), Hedychium (Kirchoff, 1997), and Scaphochlamys (Kirchoff, 1998).
Hemiorchis and Gagnepainia are small genera of the Zingiberaceae, in the subfamily Zingiberoideae tribe Globbeae (Kress et al., 2002). These two genera are closely related to each other, and molecular phylogenetic analyses indicate that they form a monophyletic group (Kress et al., 2002; Williams et al., 2004; Cao et al., 2019). Earlier treatments considered a single genus, Hemiorchis Kurz, until Schumann (1904) separated them into two genera sharing a common morphology in leaf shape, inflorescence, fruit, and pollen (Williams et al., 2004). The most important common character in these two genera is the existence of a raised median line along the central region of the labellum terminating as a pointed tip (central carina with apical projection) (Williams et al., 2004; Figures 2, 3). Although this was not explicitly mentioned by the authors, the central carina with apical projection in the labellum might represent the lost “fifth staminode” in Zingiberaceae, corresponding to an outer staminode, which is integrated in the same middle position of the labellum of Costaceae, the sister group to Zingiberaceae (Kress, 1990: Figure 1C). In other developmentally investigated species (Kirchoff, 1997, 1998; Fukai and Udomdee, 2005; Box and Rudall, 2006; Song et al., 2007), no traces of the fifth staminode were observed, and the homology of the appendage on the labellum of Hemiorchis and Gagnepainia remains unclear. Therefore, the observation of the floral development in these two genera is necessary to identify the carina morphologically.
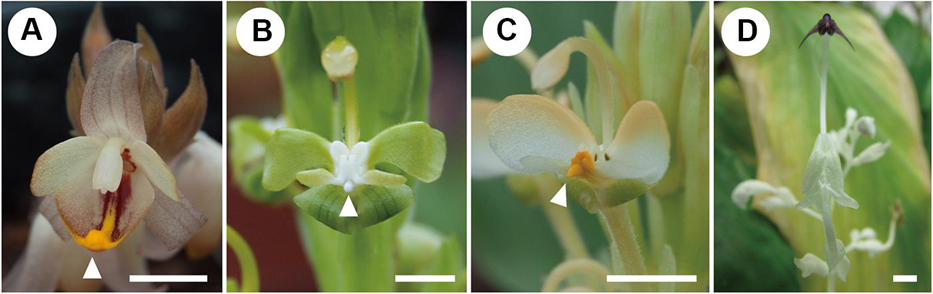
Figure 2. Mature flowers of investigated species. (A) Hemiorchis burmanica. (B) Gagnepainia harmandii. (C) Gagnepainia godefroyi. (D) Globba geofrayii. White arrows indicate the position of central carina with apical projection. Scale bars = 5 mm.
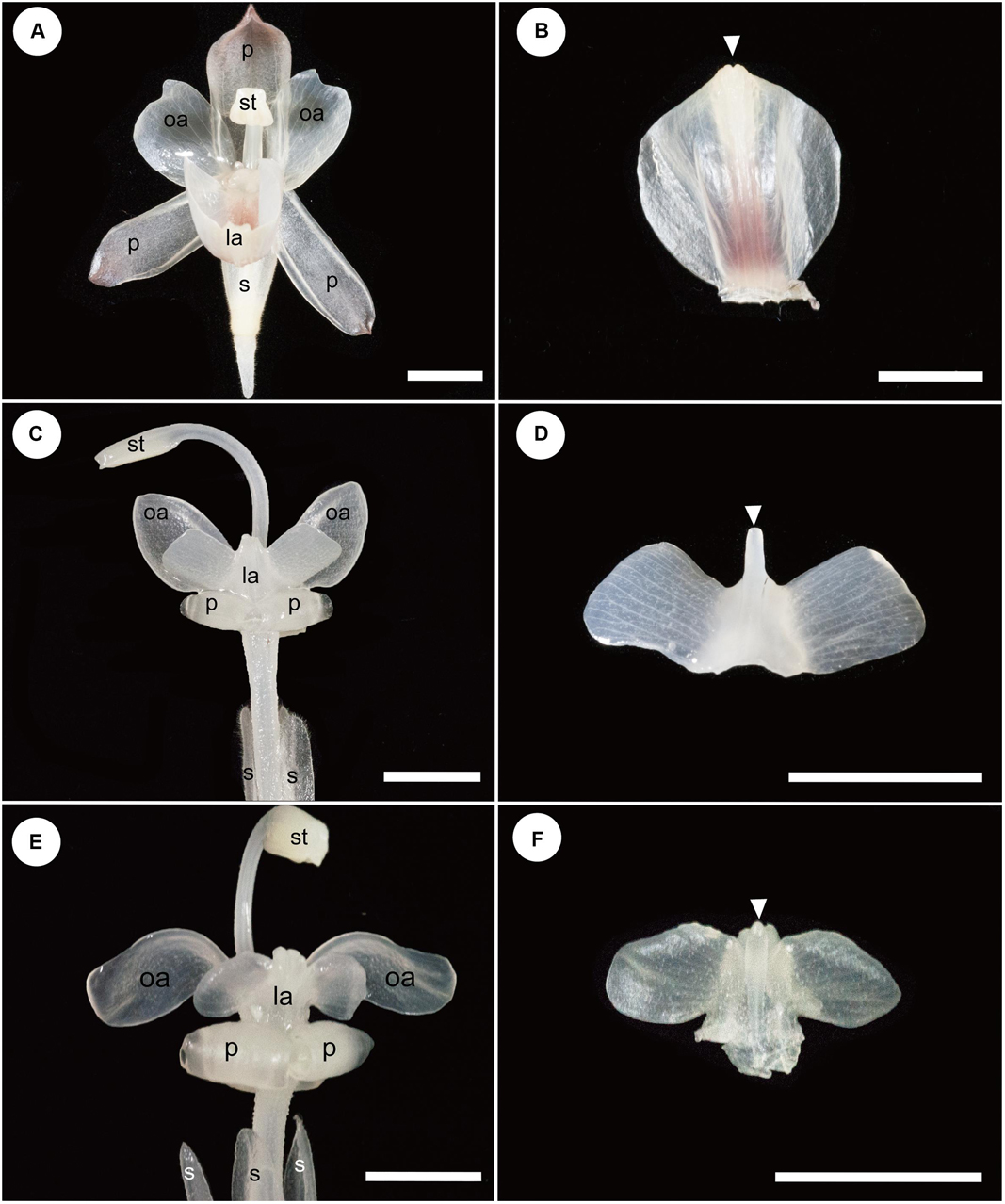
Figure 3. Floral and labellum morphology of Hemiorchis and Gagnepainia. The adaxial side of the labellum is shown, and the proximal end of the labellum is at the bottom of figures in panels (B,D,F). (A) Flower of Hemiorchis burmanica. (B) Labellum of H. burmanica, dissected from (A). White arrowhead points to the bilobed tip of the carina. (C) Flower of Gagnepainia harmandii. (D) Labellum of G. harmandii., dissected from (C). White arrowhead points to the long projection of the carina. (E) Flower of G. godefroyi. (F) Labellum of G. godefroyi, dissected from (E). White arrowhead points to the short projection of the carina. Two additional projections are formed on the abaxial side of the labellum. ca; carina of labellum; ia, inner androecial element (staminode); la, labellum; oa, outer androecial element (staminode); p, petal; s, sepal; st, stamen. Scale bars = 5 mm.
In this study, we observed the detailed floral development in Hemiorchis and Gagnepainia, in particular focusing on the development of the labellum. We also observed the development of the labellum in a selected number of species of Globba and compared the results with those of Hemiorchis and Gagnepainia. Hemiorchis and Gagnepainia are sister to Globba (including the genus Mantisia) forming the tribe Globbeae (Williams et al., 2004). An earlier floral developmental study by Box and Rudall (2006) on two species of Globba did not show any evidence of the fifth staminode. Globba, however, is the third largest genus of the Zingiberaceae and includes ∼100 species (Kress et al., 2002). Therefore, the morphological variation of flowers within the genus is inferred to be large and may include some variation in the labellum development. Through the observation of the floral development of Globbeae, we examine the potential existence of the fifth staminode in the flower of Zingiberaceae. We also aim to investigate the evolution of labellum morphology in Globbeae, which contributes to elucidate the floral evolution of the whole family, Zingiberaceae.
Materials and Methods
Plant Material
Living plants of H. burmanica Kurz, Gagnepainia harmandii (Baill.) K.Schum., and Gagnepainia godefroyi (Baill.) K.Schum. were collected at the Royal Botanic Garden Edinburgh (RBGE). All collected materials were fixed for direct or scanning electron microscopy (SEM) observation. Voucher spirit specimens of these three species are preserved in Kanagawa University and RBGE.
Floral materials of eight species of Globba were collected at RBGE for SEM observation: Globba atrosanguinea Teijsm. & Binn., Globba fasciata Ridl., Globba geoffrayi Gagnep., Globba marantina L., Globba paniculata Valeton., Globba pendula Roxb., Globba racemosa Sm., and Globba siamensis (Hemsl.) Hemsl. Voucher spirit specimens of the Globba species are preserved at RBGE (Table 1).
Fixation for Observation
The materials of H. burmanica, G. harmandii, and G. godefroyi were fixed in formalin–acetic acid–alcohol (FAA) containing less ethanol and formalin than usual (absolute ethanol, 30%/water, 62%/glacial acetic acid, 5%/formalin, 3%), and those of Globba were fixed in standard FAA (absolute ethanol, 50–70%/glacial acetic acid, 5%/and formalin, 5–7%). The samples were fixed for at least one night at room temperature, and then, FAA was replaced with 70% ethanol for the preservation of samples.
Scanning Electron Microscopy Observations
Preparation for SEM observation involved dissecting the fixed materials at various developmental stages with tweezers and a micromanipulator (MM-333, Narishige, Japan) under a stereoscopic optical microscope.
The dissected tissues were dehydrated in an ethanol series, after which the ethanol was replaced with isoamyl acetate or acetone, critical point dried with a CO2 critical point dryer (JCPD-5, JOEL, Japan and K850, Leica, Germany), and coated with Pt/Pd using a sputter coater (Ion Sputter E-1030, Hitachi, Japan and K575x, Quorum, United Kingdom).
The coated materials were observed with SEM (S3400, Hitachi, Japan and Supra 55VP, Zeiss, Germany) at 5 kV.
Results
The Floral Morphology of Hemiorchis and Gagnepainia
The results of observations on the mature flowers of H. burmanica, G. harmandii, and G. godefroyi are shown in Figure 3.
A mature flower of H. burmanica has three sepals fused at the base and three petals (Figure 3A). The flower also contains two petaloid staminodes in an outer whorl and a cymbiform labellum and one fertile stamen with a long filament in an inner whorl (Figure 3A). Note that the style is slender and contained within the abaxial furrow of the filament, which is not clear in the figure. The unfolded labellum is rhomboid and has two upright wings (Figure 3B). The central line of the labellum is raised (=carina), and the tip of the carina is slightly bilobed (Figure 3B, white arrowhead).
A mature flower of G. harmandii has three sepals fused at the base and three curled petals, although one of them is hidden behind the other two petals (Figure 3C). The flower also contains two petaloid staminodes in an outer whorl and a butterfly-shaped labellum and one sterile stamen with a long filament in an inner whorl (Figure 3C). The style is slender and contained within the abaxial furrow of filament, which is not shown in the figure. The labellum is butterfly shaped and has two lateral wings (Figure 3D). A carina is formed along the central line of the labellum, terminating with a long projection (Figure 3D, white arrowhead).
The floral structure of G. godefroyi is very similar to that of G. harmandii (Figure 3E), while the morphology of the labellum is different (Figure 3F). The labellum of G. godefroyi has also two lateral wings, but their shapes are different from those of the labellum of G. harmandii. A carina is also formed along the central line of the labellum, but its distal projection is much shorter than that of G. harmandii (Figure 3F, white arrowhead). In addition, there are two more projections on the abaxial side of the carina, which are not found in G. harmandii.
The Floral Development of Hemiorchis burmanica
At a very early developmental stage, a dome-shaped elliptical floral primordium is initiated in the axil of a bract primordium (Figure 4A). Next, three sepal primordia are initiated sequentially; the lateral sepal lobes emerge first and precede the adaxial median sepal lobe, which lags behind in growth. Simultaneously, an adaxial and abaxial common primordium are also initiated (Figure 4B). At a little later developmental stage, the two common primordia connect laterally and form a ring primordium, while the adaxial and abaxial portions can be still identified (Figure 4C). The common primordia divide the flower in two unequal halves. Next, each common primordium rapidly differentiates in the petal and androecium parts; the abaxial common primordium gives rise to the lateral inner staminodial primordia and the two abaxial petal primordia, while the adaxial common primordium gives rise to the adaxial petal, two lateral outer staminodial primordia, and the fertile stamen (Figure 4D). The stamen primordium differentiates into a massive anther, and the petal and sepal primordia also develop further (Figure 4E). The two outer lateral staminodes are hidden below the large anther. The two anterior lateral staminodes are confluent (Figure 4E). At this stage, the only possible evidence of an abaxial outer androecial primordium exists, as a weak bump between the inner androecial primordia (Figures 4D,E, white arrowhead). The bump, however, could be regarded as the boundary portion between the inner androecial primordia, which is raised by the distal ends of both primordia (see section “Discussion” for details). The sepal primordia develop further and cover the floral apex at a little later stage (Figure 4F).
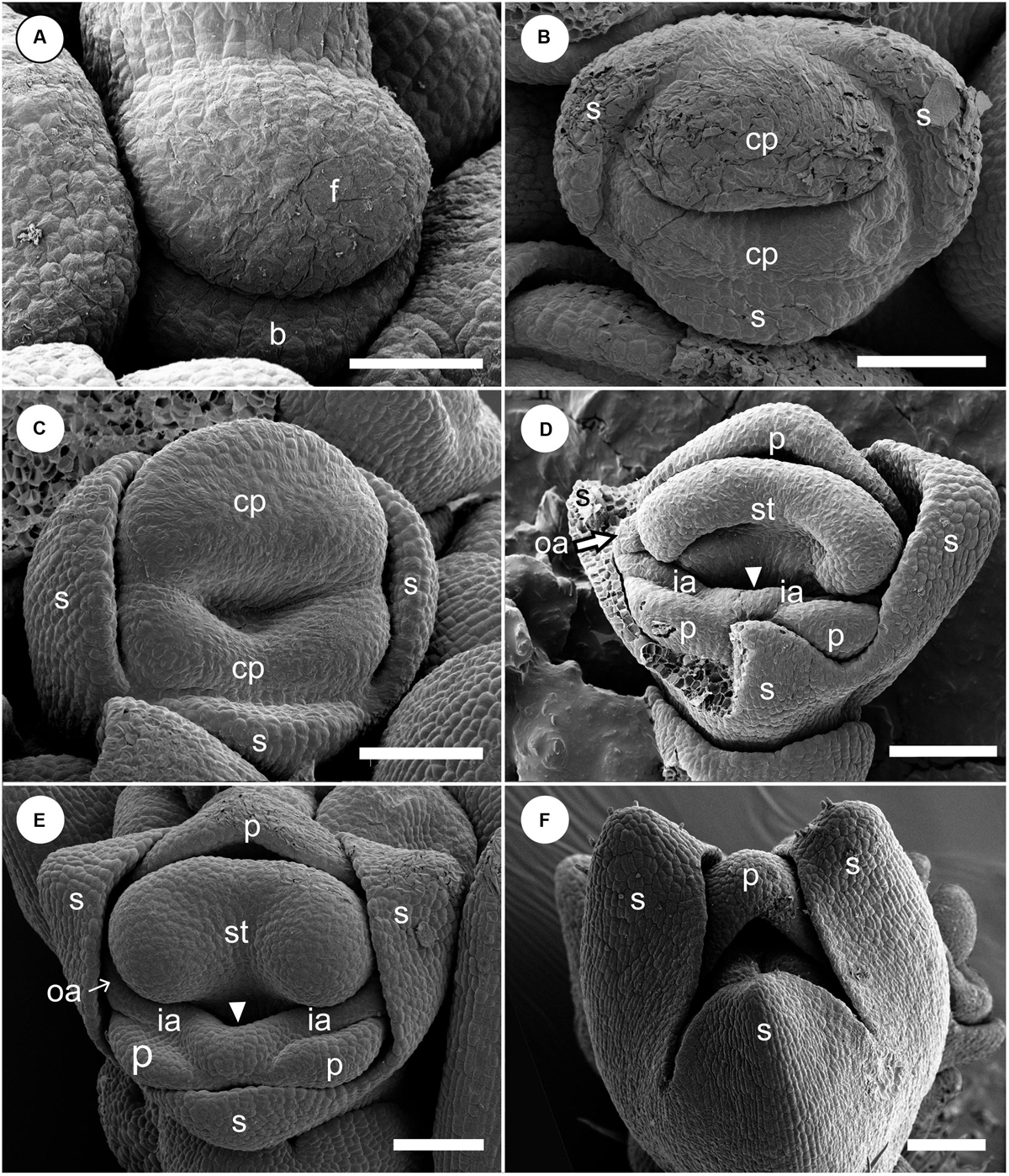
Figure 4. Early floral development of Hemiorchis burmanica. (A) Flower at the earliest stage. Dome-shaped floral bud (f) and bract (b) are initiated. (B) Three sepal primordia (s) and adaxial and abaxial common primordia (cp) are initiated. (C) Two common primordia are fused and form a ring primordium. Three sepals develop. (D,E) Petal (p), stamen (st), and two outer androecial primordia (oa) develop from the adaxial portion of the ring primordium, while two petals and inner androecial primordia develop from the abaxial portion. Note that there is a bump between the inner androecial primordia (ia), which may not be an abaxial outer androecial primordium (see text for details). (F) Three sepal primordia develop and cover the floral apex. b, bract primordium; cp, common primordium; f, floral bud; ia, inner androecial primordium (staminode primordium); oa, outer androecial primordium (staminode primordium); p, petal primordium; s, sepal primordium; st, stamen primordium. Scale bars = (A) 50 μm, (B–F) 100 μm.
We removed sepals and petals from young flowers to observe later developmental stages (Figure 5). A pistil primordium appears centrally and is adaxially enclosed by the differentiating stamen primordium. A labellum is formed by the common uplift of the two abaxial staminodes (Figure 5A). Note that there is a slight depression at the tip of the labellum, which indicates that the labellum is formed by the confluence of the two inner androecial primordia. The outer androecial primordium, if it exists, is not conspicuous (see section “Discussion” for details). The stamen differentiates two thecae, while a concavity develops on the pistil primordium, representing a stigmatic slit (Figure 5B). The style elongates and the lateral sides of the labellum expand at later developmental stages (Figure 5C). The development of the lateral outer androecial primordia is delayed, as they remain basally connected with the labellum. As the labellum develops further, the outer androecial primordia become independent from the labellum and extend in size; the style elongates and is tightly squeezed between the thecae of the adaxial stamen (Figure 5D). Finally, the labellum covers the abaxial side of the flower, and the outer lateral androecial primordia elongate along the sides of the fertile stamen to become adaxial petaloid staminodes (Figures 5E,F). Abaxially, the carina also becomes visible as a faint vertical projection (Figures 5E,F, white arrowheads).
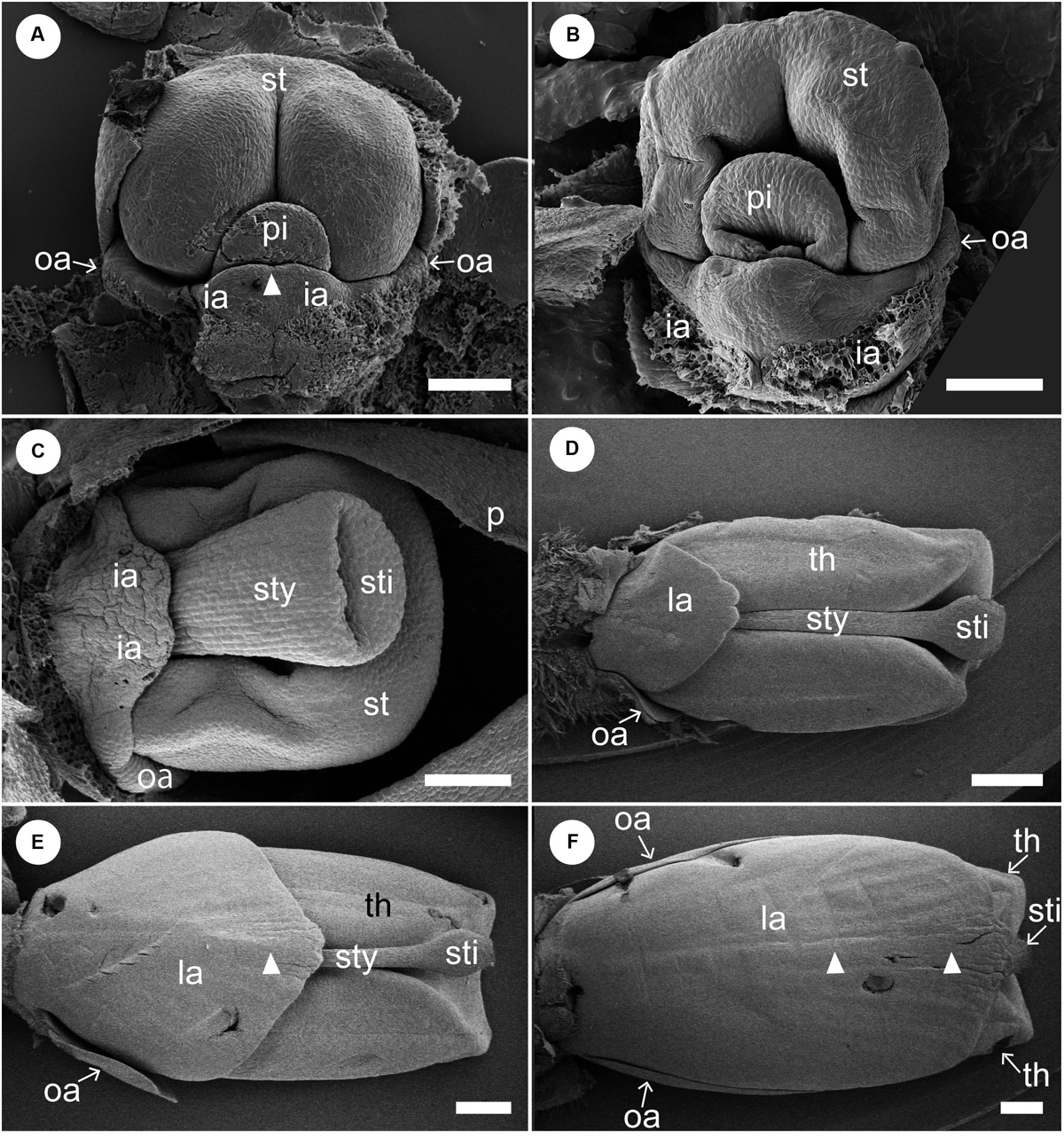
Figure 5. Later floral development of Hemiorchis burmanica (continued). Petals and sepals are removed. (A,B) Flowers at a mid-developmental stage. Two inner androecial primordia (ia) develop and are fused to form a labellum. Note that the tip of the labellum is emarginate (white arrowhead). Outer androecial primordia (oa) do not develop. The stamen primordium (st) develops and encloses the style of the pistil (sty). (C) The style elongates and a stigma is differentiated. The inner androecial primordia (labellum primordium) develop further, and outer androecial primordia are still small. The stamen develops thecae. (D) Flower at a late stage. The labellum, formed from two inner androecial primordia, and outer androecial primordia expand in size. The style elongates and is tightly enclosed within the thecae. Note that a carina primordium is secondarily initiated along the central line (white arrowhead). (E,F) The outer androecial primordia develop into petaloid wing-like staminodes. The labellum grows further and covers the abaxial side of the stamen. Note that there is a slight bump in the central portion of the labellum (white arrowheads). ia, inner androecial primordium or labellum; la, labellum; oa, outer androecial primordium or labellum petaloid staminode; st, stamen primordium; sti, stigma; sty, style; th, thecae. Scale bars = (A–C) 100 μm, (D–F) 500 μm.
We also observed the development of the adaxial side of labellum between a middle and late developmental stage corresponding to Figures 5C–F (Figure 6). At a developmental stage between Figures 5C,D, there is still no trace of a bump visible along the central line of the labellum on the adaxial side, while a slight projection is observed at the tip of labellum (Figure 6A). At a later developmental stage, a central carina is initiated, and a new lobe is initiated at the tip of carina (Figure 6B). As the labellum develops further, the central carina also expands, and its tip is still separated from the labellum as a lobe (Figure 6C). At a slight later developmental stage, the central carina expands further, and its tip is fused with the tip of the labellum (Figure 6D). A ridge develops along the central line (black arrowhead) and divides higher up in a groove with two parallel ridges. The labellum at these developmental stages (Figures 6C,D) corresponds almost to that of Figure 5D. The central carina does not develop much at a later developmental stage, which corresponds to Figure 5E, although the labellum changes in size and shape (Figure 6E). At almost the same developmental stage of Figure 5F, the central carina distinctly changes its shape (Figures 6F,G). At the upper portion of the labellum, the central groove deepens, and the two ridges expand as narrow crinkly flaps (Figure 6F), although at the lower portion of the labellum, the groove is not so obvious (Figure 6G). The lower adaxial part of the labellum differentiates as a flap of tissue expanding above the gap leading to the nectaries below and is also touching similar flaps formed by the outer staminodes (Figures 6C–E,G).
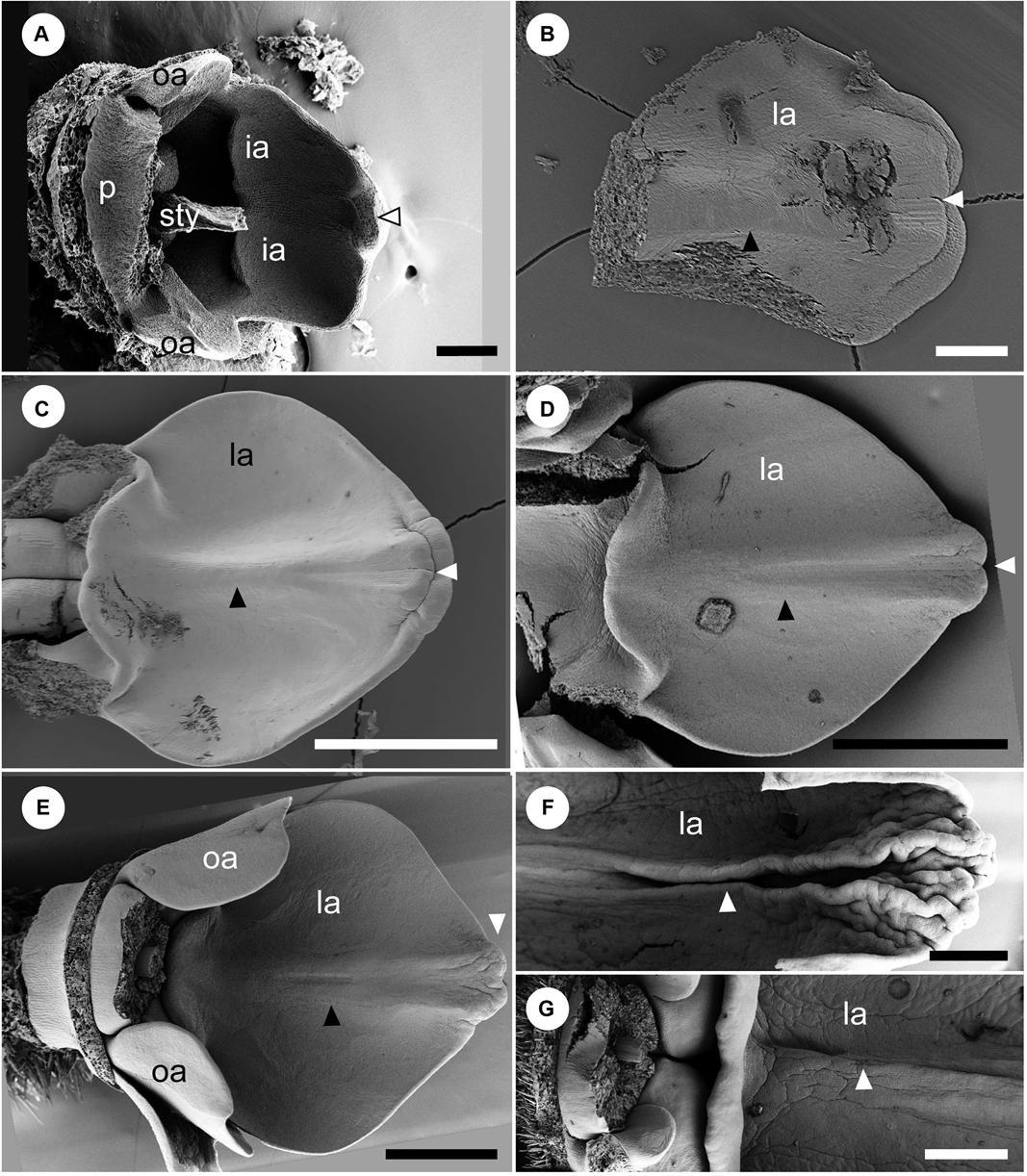
Figure 6. Later floral development of Hemiorchis burmanica (continued). The adaxial side of the labellum between middle and late developmental stages. (A) The labellum (la) at a developmental stage between Figures 5C,D. Stamen, adaxial petal, and upper portion of style are removed. Note that a central carina is not initiated, but there is a projection at the tip of the labellum (white arrowhead). (B) The labellum at a slightly later developmental stage than in (A). A carina is initiated (black arrowhead), and the tip of the labellum is horizontally bilobed (white arrowhead). (C) The labellum at almost the same developmental stage in Figure 5D. The central carina develops as a ridge (black arrowhead), and the tip of the labellum is still horizontally bilobed (white arrowhead). (D) The labellum at a slight later developmental stage than (C). The central carina is clear (black arrowhead) separating as two ridges, but the tip of the labellum is covered by the expanded inner lobe (white arrowhead). (E) The labellum at almost the same developmental stage in Figure 5E. The central carina develops further (black arrowhead) and the tip of the labellum is not horizontally bilobed (white arrowhead). (F,G) The labellum at almost the same developmental stage in Figure 5F. The (F) upper portion and (G) lower portion. The central carina (white arrowhead) is a narrow groove enclosed by two flaps and is more obvious in the upper portion. Note the three basal flaps formed by the labellum and outer staminodes, respectively. la, labellum; oa, outer androecial primordium; p, petal primordium; sti, stigma; sty, style. Scale bars = (A,B) 100 μm, (C–G) 500 μm.
The Floral Development of Gagnepainia harmandii and G. godefroyi
The early floral development of G. harmandii is almost similar to that of H. burmanica (Figure 7). The floral development begins with a dome-shaped floral primordium developing in the axil of a broad bract primordium (Figure 7A). Two sepal primordia and a two-lobed ring primordium are initiated; it separates into an adaxial and abaxial common primordium (Figure 7B). An abaxial sepal primordium eventually separates from the ring primordium but lags behind the lateral sepals (Figure 7C). An adaxial petal primordium, stamen primordium, and two lateral outer androecial primordia emerge by the subdivision of the adaxial common primordium, while an abaxial outer androecial primordium, two lateral inner androecial primordia, and two abaxial petal primordia are differentiated from the abaxial common primordium (Figures 7D,E). At these stages, the sepals enclose the whole flower (removed), and the petals progressively cover the inner portion of the flower (Figure 7F).
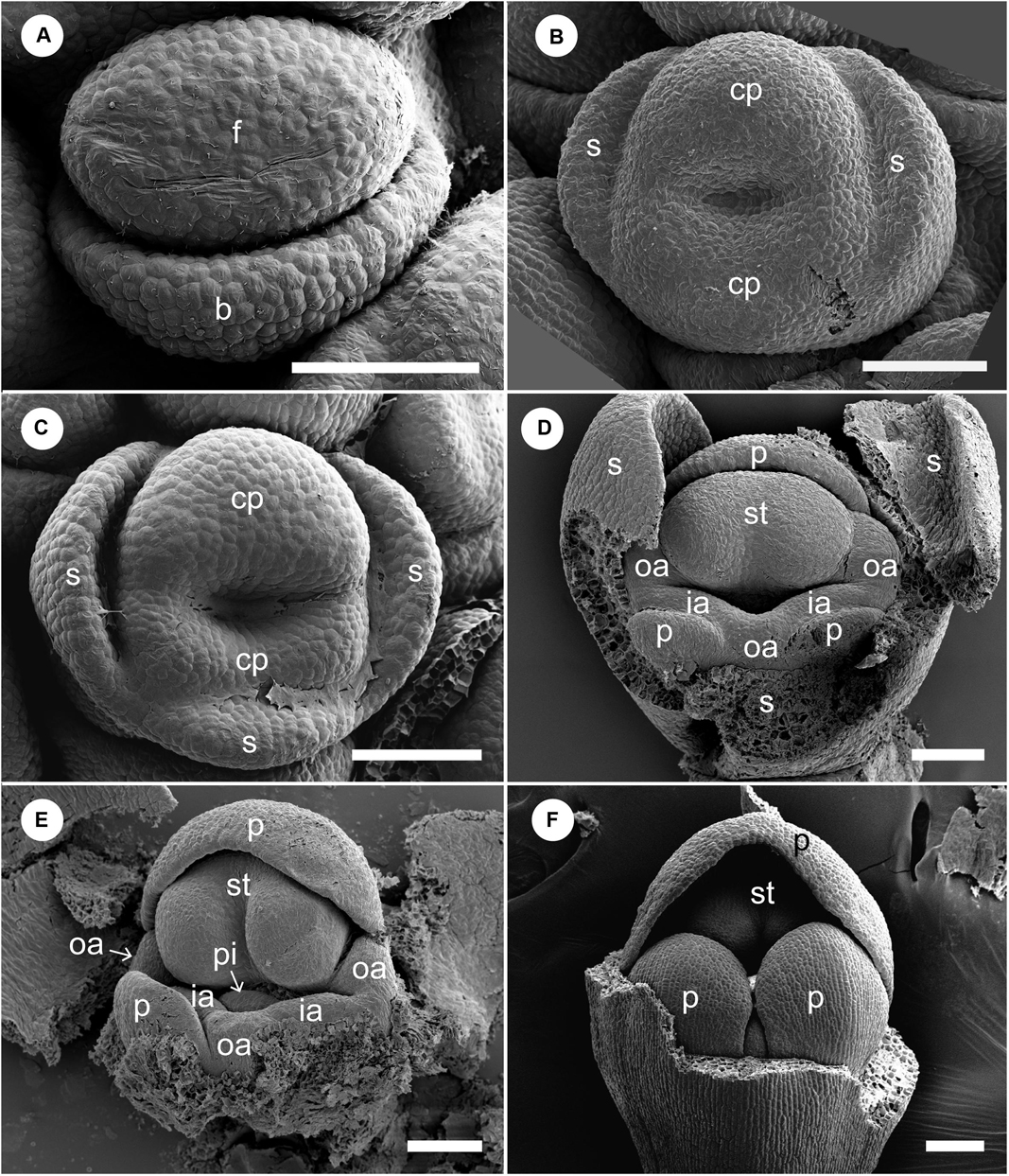
Figure 7. Floral development of Gagnepainia harmandii. (A) Flower at the earliest stage. Dome-shaped floral bud (f) and bract primordium (b) are initiated. (B) Two lateral sepal primordia (s) and ring primordium are initiated. The ring primordium can be separated into three primordia [adaxial and abaxial common primordia (cp), abaxial sepal primordia]. (C) An abaxial sepal primordium is separated from the abaxial common primordium. The abaxial common primordium divides into two portions. (D) Adaxial petal (p), stamen (st), and two outer androecial primordia (oa) are derived from the adaxial common primordium, while two abaxial petal and inner androecial primordia (ia) are derived from the abaxial common primordium. An abaxial outer androecial primordium is also initiated from the abaxial common primordium, although the boundaries with inner androecial primordia are unclear. (E) The style of the pistillate primordium appears. The abaxial outer androecial primordium develops further and the boundaries with inner androecial primordia become clear. Sepal primordia are removed. (F) The abaxial petal primordia develop and cover the abaxial side of the stamen. Sepal primordia are removed. b, bract primordium; cp, common primordium; f, floral bud; ia, inner androecial primordium (staminode primordium); oa, outer androecial primordium (staminode primordium); p, petal primordium; s, sepal primordium; st, stamen primordium. Scale bars = 100 μm.
We removed the sepals and one abaxial petal and observed the development of the inner portion of the flower (Figures 8, 9). At almost the same developmental stage of the flower in Figure 7F, a pistil primordium appears within the groove of the stamen, and each androecial primordium (two lateral outer primordia, one abaxial outer primordium, and two inner primordia) are clearly visible (i.e., the limits between these primordia are conspicuous) (Figures 8A,B). At a slightly later stage, the pistil primordium develops a stigma by the unequal development of a central depression. All androecial elements are lifted by common basal growth, but the limits between the elements are still conspicuous (Figures 8C,D). Next, the style elongates in a column-like structure with an apical stigmatic slit (Figure 9). The labellum is clearly composed of three androecial primordia; in particular, the abaxial outer androecial primordium is conspicuous and independent from the two inner androecial members. The abaxial outer androecial primordium becomes a long, narrow appendage on the labellum, and the elongated style is tightly enclosed by the anther developing a prominent connective (Figure 9D).
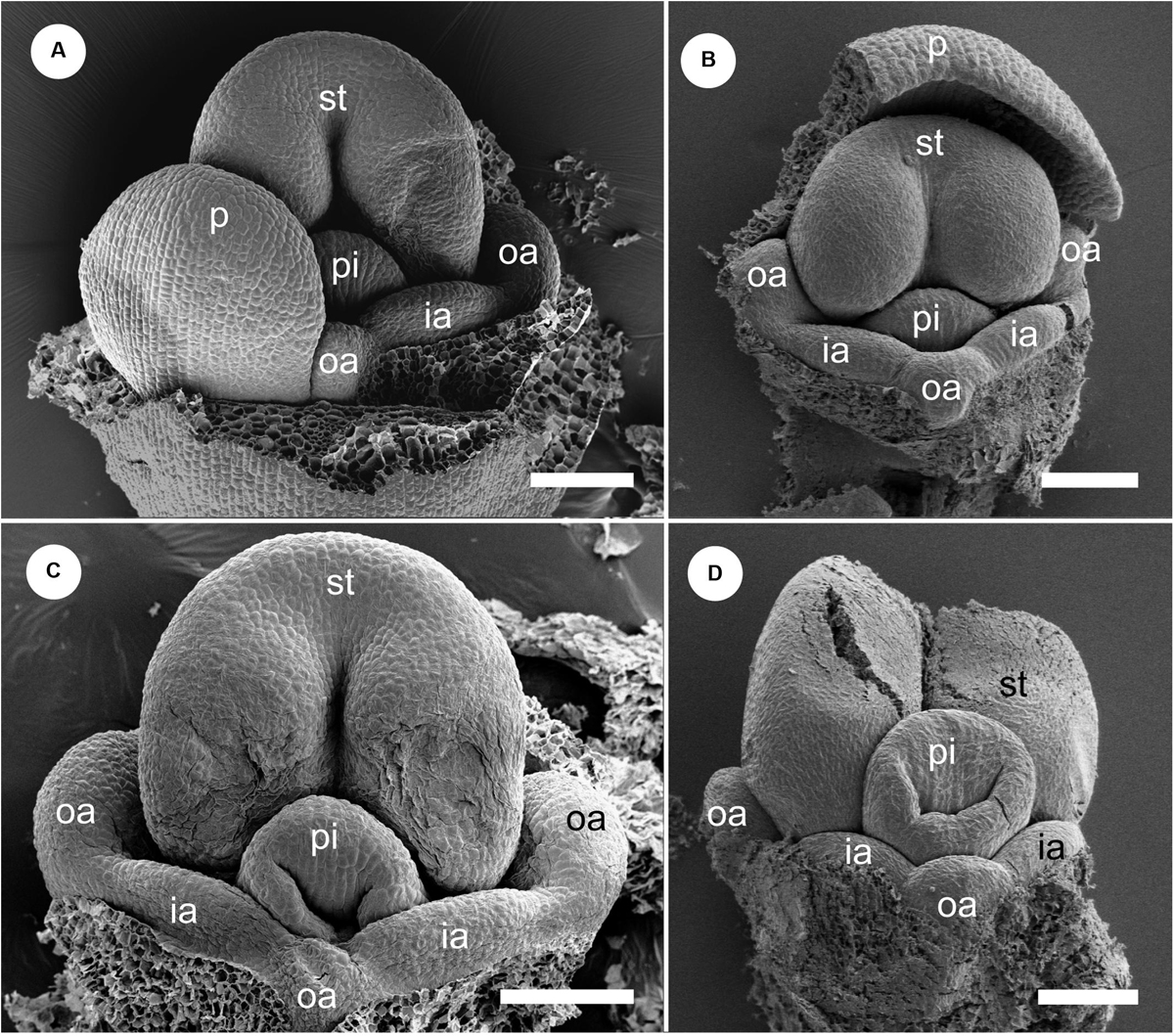
Figure 8. Floral development of Gagnepainia harmandii (continued). Petals and sepals are removed. (A,B) Flowers at almost the same developmental stage as Figure 6F (middle stage). One of the abaxial petal primordia (p) is not removed in (A). The androecial primordia are still small, but the boundaries between the abaxial outer androecial primordia (oa) are clear. (C,D) A slit develop at the top of the style (sty). The androecial primordia develop slightly, and the size of the abaxial outer androecial primordium becomes almost the same as the inner androecial primordia (ia) and outer lateral androecial primordia in (D). ia, inner androecial primordium; la, labellum; oa, outer androecial primordium; p, petal primordium; st, stamen primordium; sty, style. Scale bars = 100 μm.
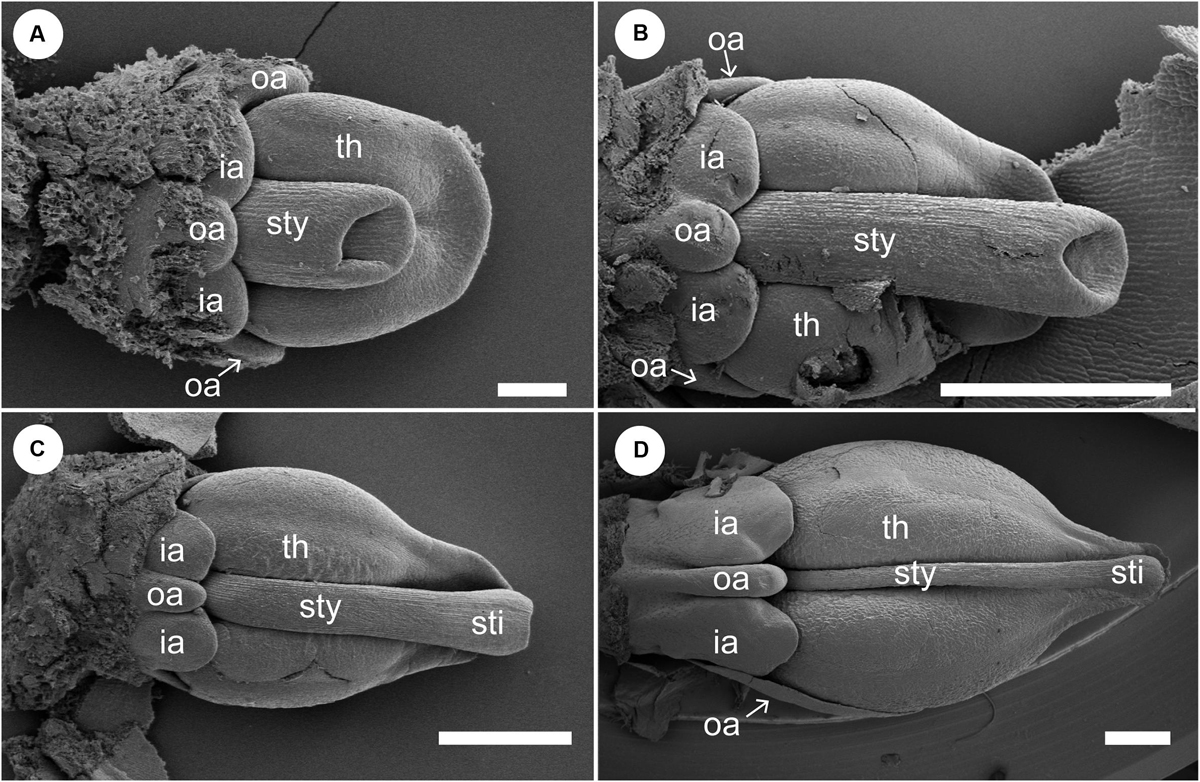
Figure 9. Floral development of Gagnepainia harmandii (continued). Petals and sepals are removed. (A,B) Flowers at a late stage. The style (sty) elongates and is enclosed within the thecae (th). The abaxial outer androecial primordium (oa) and two inner androecial primordia (ia) develop and form a labellum primordium, while the other outer androecial primordia develop along the lateral sides of the stamen. (C,D) The abaxial outer androecial primordium elongates and becomes a long carina of the labellum. The inner androecial primordia also develop into lateral wings of the labellum. The lateral outer androecial primordia develop into petaloid staminodes along the lateral sides of the stamen. The style elongates and is tightly enclosed within the thecae. ia, inner androecial primordium; oa, outer androecial primordium; th, thecae; sti, stigma; sty, style. Scale bars = 500 μm.
We observed the floral development of G. godefroyi at later developmental stages and confirmed that the labellum clearly includes the abaxial outer androecial primordium similar to G. harmandii (Figure 10).

Figure 10. Flower at late mid-developmental stage of Gagnepainia godefroyi. Petals and sepals are removed. (A) Two inner androecial primordia and three outer androecial primordia (oa), including the abaxial primordium are initiated. (B) The style elongates and is enclosed between thecae (th). Two inner androecial primordia (ia) and an outer androecial primordium develop and form the labellum primordium. Abbreviations: ia, inner androecial primordium; oa, outer androecial primordium; pi, pistil primordium; st, stamen primordium. Scale bars = (A) 100 μm, (B) 500 μm.
The Labellum Development of Globba
We found that there are two types of labellum development in the examined eight species of Globba. Therefore, the labellum development of two species, G. paniculata and G. geoffrayi, representing each type, is shown in Figure 11.
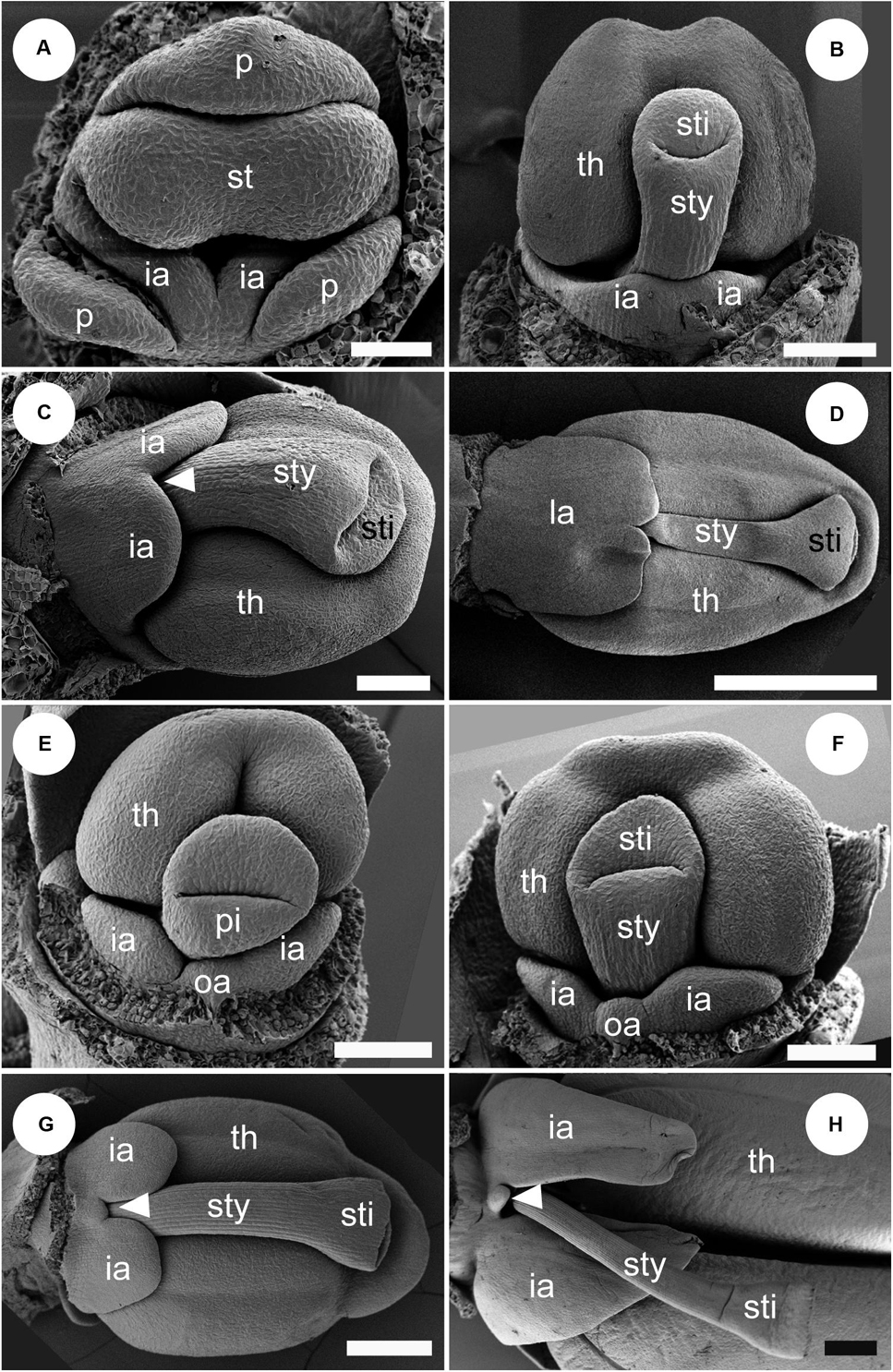
Figure 11. Labellum development of Globba. Sepals are removed in panel (A), and both sepals and petal are removed in panels (B–H). (A–D) Labellum development of Globba paniculata. (A) Two inner androecial primordia (ia) are initiated, and an outer abaxial androecial primordium (oa) is not observed. (B) The two inner abaxial staminodes develop as a broad bilobed primordium. (C) The limit between the two staminodes becomes clear as an apical notch (arrowhead). (D) The labellum grows upward and progressively covers the abaxial side of the stamen; the tip of the labellum is depressed. (E–H) Labellum development of G. geoffrayi. (E,F) Two inner androecial primordia and an outer abaxial primordium are initiated. (G,H) Two inner androecial primordia develop and an outer abaxial primordium is small, although it clearly exists. ia, inner androecial primordium; oa, outer androecial primordium; p, petal primordium; st, stamen primordium; sty, style; th, thecae. Scale bars = (A) 50 μm, (B,C,E,F) 100 μm, (D,G,H) 500 μm.
At a mid-developmental stage of the flower of G. paniculata, two inner staminode primordia are initiated (Figure 11A). As a pistillate primordium appears and its style elongates, the two inner androecial primordia are lifted by common growth and form a broad primordium (Figure 11B, labellum primordium). The boundary between the inner androecial primordia becomes clear at a slight later stage as a notch (Figure 11C, white arrowhead). Consequently, a rounded labellum is differentiated retaining the apical depression, indicating its dual nature (Figure 11D). An abaxial outer androecial primordium or its vestige was not found throughout the labellum development of G. paniculata (Figures 11A–D). The other seven Globba except for G. geoffrayi showed almost the same labellum development.
Two inner androecial primordia and an abaxial outer androecial primordium are initiated at a mid-developmental stage of the flower in G. geoffrayi when the style appears, and the fertile stamen primordium differentiates anther tissue (Figure 11E). Immediately afterward, the style elongates and is enclosed between the thecae, and the limits on both sides of the abaxial outer primordia are clear (Figure 11F). As the style elongates, the inner staminodes also develop, although the abaxial outer staminode ceases its development (Figures 11G,H). Compared with the inner staminodes, the abaxial outer staminode is very small but still exists even at a late developmental stage. G. geoffrayi is the only species showing this type of labellum development among the eight examined Globba species.
Discussion
The Early Floral Development of Hemiorchis and Gagnepainia Focusing on Common Primordia
Hemiorchis and Gagnepainia are small poorly understood genera in Zingiberaceae (Kress et al., 2002). We revealed the floral development of both genera for the first time in this study. The early floral development of both genera (Figures 4A–E, 7A–E) is generally consistent with that of other genera in Zingiberaceae (Kirchoff, 1997, 1998; Fukai and Udomdee, 2005; Box and Rudall, 2006; Song et al., 2007).
Both flowers of Hemiorchis and Gagnepainia have a characteristic ring primordium at an early stage that can be separated into an abaxial and adaxial common primordium (Figures 4C, 7C), although all previous studies on the floral development in the family concluded that it can be separated into three common primordia, an adaxial and two lateral abaxial primordia (Kirchoff, 1997, 1998; Fukai and Udomdee, 2005; Box and Rudall, 2006; Song et al., 2007). We interpret that the lateral abaxial common primordia originated from a single primordium because we observed the flowers at critical stages just before the ring primordium is formed (Figures 4B, 7B). A single abaxial common primordium can be identified at this stage, and even an abaxial sepal primordium appears closely connected to the common primordium in Gagnepainia. It would be necessary to focus on the early floral development of other members in Zingiberaceae to conclude whether the single abaxial common primordium is a distinct character in the floral development of Hemiorchis and Gagnepainia or a common character in Zingiberaceae.
The Labellum Development in Globbeae
The existence of an abaxial outer androecial primordium at an early floral developmental stage, the primordium of a “fifth” staminode, has been controversial in Zingiberaceae. Kirchoff (1997, 1998) and Song et al. (2007) recognized the existence of an abaxial outer androecial primordium at early floral developmental stages and concluded that it rapidly ceased to grow and was aborted at later stages in Alpinia oxyphylla (Song et al., 2007), Hedychium gardnerianum (Kirchoff, 1997), and Scaphochlamys kunstleri (Kirchoff, 1998). Fukai and Udomdee (2005) mentioned that there are only two inner androecial primordia on the abaxial side of the flower at an early floral developmental stage in Curcuma alismatifolia, and Box and Rudall (2006) found that it was not clear whether the abaxial outer androecial primordium is initiated and subsequently aborted during development or is absent entirely in Globba spathulata and Globba cernua. Although the morphology of the flower at early developmental stages is almost similar in all studies, their interpretations are different. In this study, we also observed a clear bump in a median position between two inner androecial primordia during early floral development in Hemiorchis and Gagnepainia, which could represent the abaxial outer androecial primordium (Figures 4D,E, 7D,E).
However, we consider that the small bump on the young flower of Hemiorchis may not be the abaxial outer androecial primordium but just the boundary portion between the inner androecial primordia, which is raised by the distal ends of two inner androecial primordia, while the bump in the early flower of Gagnepainia is clearly the abaxial outer androecial primordium. It is indubitable that the bump in Gagnepainia is the abaxial outer androecial primordium because it subsequently grows into the central portion of the labellum at later developmental stages (Figures 7–10).
It would be rather difficult to identify the bump in Hemiorchis because there is no vestige of it at later developmental stages but just a depression at a median position of the labellum (Figure 5). Kirchoff (1997, 1998) interpreted a similar bump in Scaphochlamys and Hedyosmum as the abaxial outer androecial primordium, which rapidly ceased to grow and became aborted as a depression at a median position of the labellum. As Hemiorchis showed a similar development and if the interpretation about Scaphochlamys and Hedyosmum is correct, it also initiates the abaxial outer androecial primordium at first and the primordium is rapidly aborted. We observed, however, the labellum development of G. geoffrayi and found that the abaxial outer androecial primordium ceases to grow eventually, but it clearly remains visible between two abaxial inner androecial primordia (Figures 11E–H). This observation suggests that, if the abaxial outer androecial primordium was once initiated and rapidly ceased to grow at a later developmental stage in Hemiorchis, Scaphochlamys, and Hedyosmum, as Kirchoff (1997, 1998) suggested, the median portion of the labellum would be more depressed as is the case in G. geoffrayi, and the labellum would be clearly divided into two lobes without a intermedial bump.
In case that the abaxial outer androecial primordium is not initiated, as in other investigated species of Globba (Figures 11A–D), and the two inner androecial primordia occupy its position, a labellum with only an apical depression is formed as in most members of Zingiberaceae. We concluded that the labellum development of Hemiorchis (Figures 4E, 5A–D), Scaphochlamys, and Hedyosmum (Kirchoff, 1997, 1998) should be essentially consistent with that of other genera in Zingiberaceae including Globba (Figures 11A–D; Kirchoff, 1997, 1998; Fukai and Udomdee, 2005; Box and Rudall, 2006; Song et al., 2007). That is, the labellum is clearly composed of two inner staminodes and contains no outer staminode, including a “fifth” staminode. The carina of the mature labellum in Hemiorchis (Figure 3B) should be considered as a secondary organ because it is initiated and develops only later on the adaxial side of the labellum (Figure 6). In particular, an independent lobe is observed at the tip of the labellum on the adaxial side during early labellum developmental stages (Figures 6B,C), which strongly suggest that the central carina is not derived from the abaxial outer androecial primordium but is a structure secondarily derived from the adaxial side of the labellum.
The labellum development of Gagnepainia is distinct in Zingiberaceae on the other hand. In G. harmandii, the abaxial outer primordium is clearly initiated (Figure 8) and develops along with two inner androecial primordia to form the labellum (Figure 9). The carina of the mature labellum in G. harmandii (Figure 3D) is certainly derived from the abaxial outer primordium, which corresponds to the abaxial outer staminode. In the other species of Gagnepainia, G. godefroyi, the abaxial outer primordium is clearly initiated and develops in a similar way (Figure 10), which indicates that the carina of this species (Figure 3F) is also equivalent to the abaxial outer staminode. We can conclude that the labellum of Gagnepainia contains the abaxial outer staminode, the “fifth” staminode, which has been considered to be lost or strongly reduced in the labellum of Zingiberaceae (Kress, 1990; Kress et al., 2002).
In Globba, we found two types of labellum development (Figure 11). The abaxial outer androecial primordium is not initiated and not included in a labellum of seven of eight observed Globba species (Figures 11A–D), which is consistent with the previous study on the floral development of G. cernua and G. spathulata (Box and Rudall, 2006). However, the labellum development of G. geoffrayi is distinguished from the dominant type. In G. geoffrayi, the abaxial outer androecial primordium is initiated and ceases to grow, but it clearly persists between the two abaxial inner androecial lobes for a considerable part of the development (Figures 11E–H). The labellum is an intermediate type between that of Gagnepainia and the other species in Zingiberaceae including Hemiorchis and Globba.
We can conclude that the development of the fifth staminode is a clear case of heterochrony (cf. Ronse De Craene, 2018), showing a gradual transition between a fully developed staminode as in Gagnepainia, to a reduced staminode in G. geoffrayi, and further to a complete loss of the staminode as in other Zingiberaceae. The loss of the staminode is clearly linked to a progressive termination of initiation of the outer abaxial staminode.
The Evolution of Labellum Morphology in Globbeae
We mapped the labellum development observed in this study and in Box and Rudall (2006) on a simplified phylogenetic tree of the tribe Globbeae based on Cao et al. (2019) (Figure 12). We added the other clades, viz., the tribe Zingibereae, subfamily Alpinioideae, and the genera Tamijia and Siphonochilus based on Kress et al. (2002) and Cole (2018), and mapped the labellum development data from previous studies (Kirchoff, 1997, 1998; Fukai and Udomdee, 2005; Song et al., 2007).
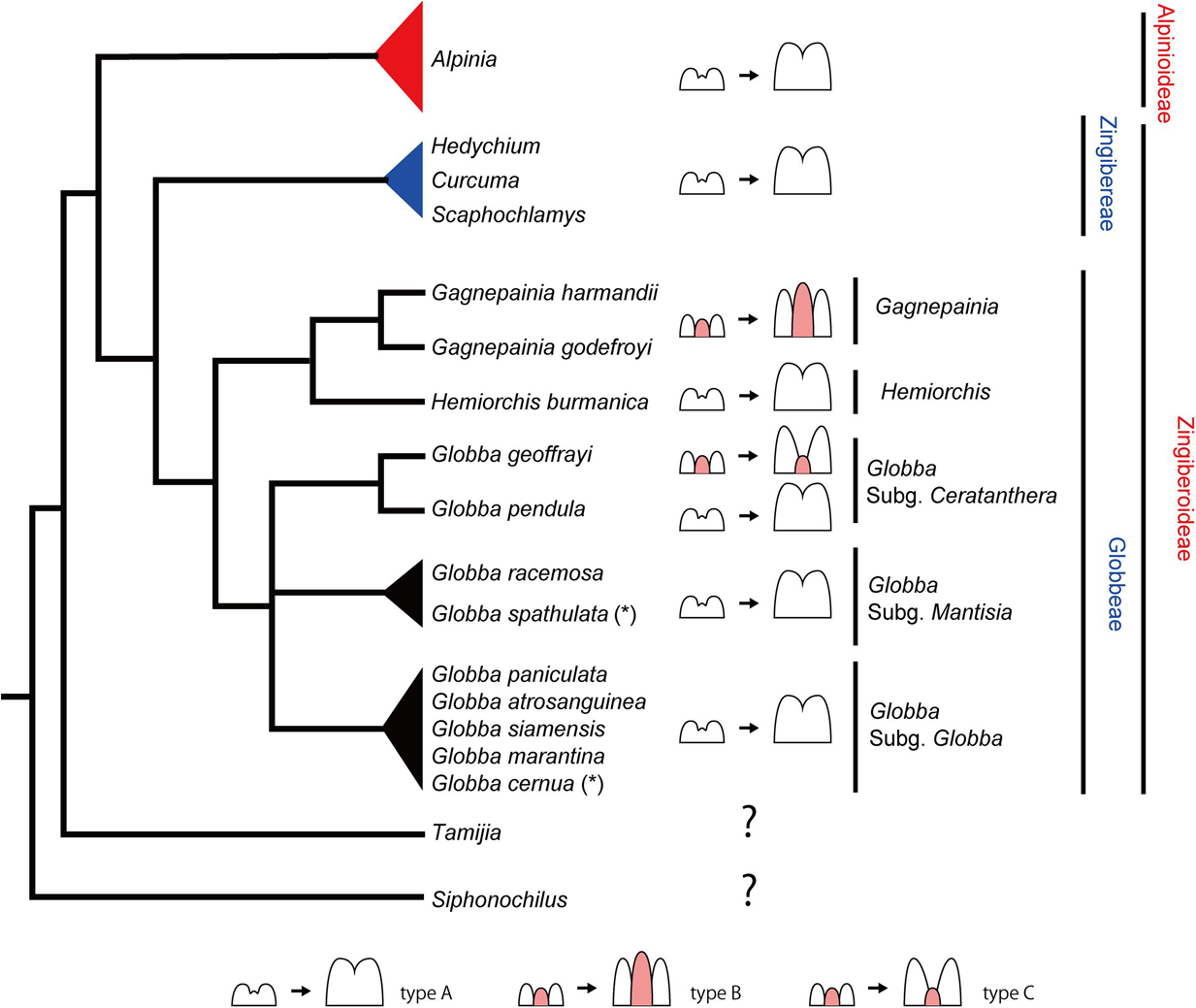
Figure 12. The evolution of labellum development in Globbeae. Mapping the labellum development on a simplified phylogenetic tree of the tribe Globbeae based on Cao et al. (2019). The phylogenetic position of Zingibereae, Alpinioideae, Tamijia, and Siphonochilus are based on Kress et al. (2002) and Cole (2018). Pink highlighted portion in each schematic diagram of labellum development represents the missing fifth staminode. Note that the small bump between bilobed primordia of the left drawing of type A may not be the fifth staminode; therefore, it is not highlighted in pink. For Globbeae, observed species in this study and two species adapted from Box and Rudall (2006) are mapped on the tree, although they do not cover all species included in the original tree of Cao et al. (2019) (some species missing in subgenus Mantisia and Globba, represented by black triangles). (*) represents a species based on Box and Rudall (2006). The references for the labellum development in Zingibereae and Alpinioideae are as follows: Alpinia, Song et al. (2007); Curcuma, Fukai and Udomdee (2005); Hedychium, Kirchoff (1997); and Scaphochlamys, Kirchoff (1998).
While we do not have observations from all species included in the phylogenetic tree of Globbeae (Figure 12, black triangles), that is, not all species from subgenera Mantisia and Globba were observed, observations mapped onto this phylogenetic tree provide a good starting point to discuss the evolution of labellum morphology in Globbeae and do reveal information on evolutionary patterns of labellum development across the tribe.
The labellum comprising only two inner staminodes (type A) is the most common state across the tribe. Hemiorchis and Gagnepainia form a clade within Globbeae, and the labellum that contains a well-developed fifth staminode (type B) is only found in Gagnepainia. The genus Globba is sister to the clade containing all Hemiorchis and Gagnepainia species, with the monophyletic Globba subgenus Ceratanthera resolved as the earliest diverging lineage within Globba (Williams et al., 2004). Within subgenus Ceratanthera, while G. pendula has the two staminode labellum as most other species in Globbeae, a labellum that includes a remnant of the fifth staminode (type C) has evolved independently in G. geoffrayi. G. geoffrayi also differs from all other species of Globba in distinct floral features, including the unusual development of anther appendages (Cao et al., 2019). All other species investigated, including all species of subgenus Globba, develop a type A labellum.
It is not clear whether the strongly developed carina of Gagnepainia (type B) is a retained plesiomorphic condition (comparable to the well-developed staminodes of other Zingiberales, for example Costaceae) or a secondary reversal from a staminode in the process of reduction. This evolutionary trend is comparable to staminode evolution in the Primuloid clade, with a different extent of development of the antesepalous staminodial whorls (Ronse De Craene, 2010, 2018). If type B in Gagnepainia is the retained plesiomorphic condition, it is only retained in G. geoffrayi as the remnant staminode (type C) in the genus Globba. Otherwise, the type C labellum evolved independently from Gagnepainia, which is a neoteny of type B. The comparative observation of labellum development of Siphonochilus and Tamijia, which are the most basal lineages in Zingiberaceae (Kress et al., 2002), and further species in Globbeae is necessary to determine whether the existence of a fifth staminode in Globbeae is plesiomorphic or not. As the fifth staminode exists and is integrated into the labellum of the flower of Costaceae (Kress, 1990), the development of a fifth staminode and integration into the labellum in Gagnepainia and G. geoffrayi may be its retention in the basal lineages of Globbeae. It is also important to extend the observation of labellum development to the tribe Zingibereae and subfamily Alpinoideae. In these taxa, although only type C has been observed in the previous studies (Figure 12), there remains the possibility of the existence of type A and/or type B as observed in Globbeae.
The retention or recovery of the fifth staminode and its heterochronic shift should be linked with the spatial constriction within the flower bud. Iwamoto et al. (2003, 2015) suggested that the initiation and development of floral organs could be suppressed by mechanical pressures on the floral meristem. In fact, the flower buds at early stages in Zingiberaceae including Globbeae observed in this study are tightly packed insides the sepals (e.g., Figures 4D,E, 7D,E), and there could be a strong mechanical pressure between the different elements of the abaxial common primordium, including the labellum and other floral organs, which might cause the suppression of initiation of the abaxial outer androecial primordium and its heterochronic shift (Ronse De Craene, 2018). Detailed anatomical observations, in particular on the longitudinal sections of flower bud at late developmental stages, are necessary to elucidate if there are clear mechanical pressures on the abaxial common primordium.
Conclusion
The floral development of Hemiorchis and Gagnepainia at early stages before the onset of labellum development is generally consistent with that of other members in Zingiberaceae. The fifth staminode develops as part of the labellar carina in Gagnepainia, while it is generally lost in Zingiberaceae. The primordium of the fifth staminode is not initiated and lost in the labellum of Hemiorchis. As a result, the carina in the labellum of Hemiorchis is a secondary organ. The fifth staminode also develops in G. geoffrayi, but it is not initiated in all other species of Globba investigated. While the staminode becomes the conspicuous carina of the labellum in Gagnepainia, it ceases to grow and remains small even at later stages in G. geoffrayi.
The fifth staminode could be either a retained plesiomorphy or the recovery of a lost organ in the basal lineage of Globbeae. The initiation of the fifth staminode and its heterochronic shift may be linked to the spatial constriction within the flower bud. It is necessary to investigate the labellum development of other Zingiberaceae, in particular, further species in Globbeae and in the genera Siphonochilus and Tamijia, which are the most basal lineages in Zingiberaceae to understand to what extent the fifth staminode has persisted among the family.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
AI and LR had substantial contributions to the conception or design of the work, drafted the manuscript. AI and SI observed the floral morphology and development of Gagnepainia and Hemiorchis. LR and LC observed those of Globba.
Funding
This work was supported by MEXT KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas “Plant-Structure Optimization Strategy” Grant Number JP19H05358 (to AI), and by JSPS KAKENHI Grant Number JP16K18576 (to AI).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Royal Botanic Garden Edinburgh for support in collecting the materials.
References
Barrett, C. F., Specht, C. D., Leebens-Mack, J., Stevenson, D. W., Zomlefer, W. B., and Davis, J. I. (2014). Resolving ancient radiations: can complete plastid gene sets elucidate deep relationships among the tropical gingers (Zingiberales)? Ann. Bot. 113, 119–133. doi: 10.1093/aob/mct264
Box, M., and Rudall, P. (2006). Floral structure and ontogeny in Globba (Zingiberaceae). Plant Syst. Evol. 258, 107–122. doi: 10.1007/s00606-005-0395-4
Cao, L., Newman, M. F., Kirchoff, B. K., and Ronse de Craene, L. P. (2019). Developmental evidence helps resolve the evolutionary origins of anther appendages in Globba (Zingiberaceae). Bot. J. Linn. Soc. 189, 63–82. doi: 10.1093/botlinnean/boy071
Cole, T. C. H. (2018). Zingiberaceae Phylogeny Poster. Available online at: www.researchgate.net/publication/314205060_Zingiberaceae_Phylogeny_ Poster_ZPP (accessed December, 2019).
Dahlgren, R. M. T., Clifford, H. T., and Yeo, P. F. (1985). The Families Of The Monocotyledons. Structure, Evolution, and Taxonomy. Berlin: Springer-Verlag.
Fukai, S., and Udomdee, W. (2005). Inflorescence and flower initiation and development in Curcuma alismatifolia Gagnep (Zingiberaceae). Jpn. J. Trop. Agric. 49, 14–20. doi: 10.11248/jsta1957.49.14
Iwamoto, A., Izumidate, R., and Ronse De Craene, L. P. (2015). Floral anatomy and vegetative development in Ceratophyllum demersum: a morphological picture of an “unsolved” plant. Am. J. Bot. 102, 1578–1589. doi: 10.3732/ajb.1500124
Iwamoto, A., Nakamura, A., Kurihara, S., Otani, A., and Ronse De Craene, L. (2018). Floral development of petaloid Alismatales as an insight into the origin of the trimerous Bauplan in monocot flowers. J. Plant Res. 131, 395–407. doi: 10.1007/s10265-018-1022-0
Iwamoto, A., Shimizu, A., and Ohba, H. (2003). Floral development and phyllotactic variation in Ceratophyllum demersum (Ceratophyllaceae). Am. J. Bot. 90, 1124–1130. doi: 10.3732/ajb.90.8.1124
Kirchoff, B. K. (1997). Inflorescence and flower development in the Hedychieae (Zingiberaceae): Hedychium. Can. J. Bot. 75, 581–594. doi: 10.1139/b97-065
Kirchoff, B. K. (1998). Inflorescence and flower development in the Hedychieae (Zingiberaceae): Scaphochlamys kunstleri (Baker) Holttum. Int. J. Plant Sci. 159, 261–274. doi: 10.1086/297547
Kress, W. J. (1990). The phylogeny and classification of the Zingiberales. Ann. Mo. Bot. Gard. 77, 698–721. doi: 10.2307/2399669
Kress, W. J., Prince, L. M., Hahn, W. J., and Zimmer, E. A. (2001). Unraveling the evolutionary radiation of the families of the zingiberales using morphological and molecular evidence. Syst. Biol. 50, 926–944. doi: 10.1080/106351501753462885
Kress, W. J., Prince, L. M., and Williams, K. J. (2002). The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. Am. J. Bot. 89, 1682–1696. doi: 10.3732/ajb.89.10.1682
Ronse De Craene, L. (2010). Floral Diagrams: An Aid To Understanding Flower Morphology And Evolution. Cambridge, MA: Cambridge University Press.
Ronse De Craene, L. (2018). Understanding the role of floral development in the evolution of angiosperm flowers: clarifications from a historical and physico-dynamic perspective. J. Plant Res. 131, 367–393. doi: 10.1007/s10265-018-1021-1
Sass, C., Iles, W. J. D., Barrett, C. F., Smith, S. Y., and Specht, C. D. (2016). Revisiting the Zingiberales: using multiplexed exon capture to resolve ancient and recent phylogenetic splits in a charismatic plant lineage. PeerJ 4:e1584. doi: 10.7717/peerj.1584
Song, J. J., Zou, P., Liao, J. P., Tang, Y. J., and Chen, Z. Y. (2007). Floral ontogeny in Alpinia oxyphylla Miq. (Zingiberaceae) and its systematic significance. Gard. Bull. Singap. 59, 221–230.
Keywords: floral development, Globbeae, heterochrony, labellum, staminode, Zingiberaceae
Citation: Iwamoto A, Ishigooka S, Cao L and Ronse De Craene LP (2020) Floral Development Reveals the Existence of a Fifth Staminode on the Labellum of Basal Globbeae. Front. Ecol. Evol. 8:133. doi: 10.3389/fevo.2020.00133
Received: 31 December 2019; Accepted: 22 April 2020;
Published: 04 June 2020.
Edited by:
Alessandro Minelli, University of Padova, ItalyReviewed by:
Catherine Damerval, UMR8120 Génétique Quantitative et Evolution Le Moulon, FranceChelsea D. Specht, Cornell University, United States
Copyright © 2020 Iwamoto, Ishigooka, Cao and Ronse De Craene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akitoshi Iwamoto, akitoshi@kanagawa-u.ac.jp
 Akitoshi Iwamoto
Akitoshi Iwamoto Shiori Ishigooka2
Shiori Ishigooka2  Louis P. Ronse De Craene
Louis P. Ronse De Craene