Pachygenium laurense (Orchidaceae, Spiranthinae), a new orchid species from Argentina—morphological evidence and phylogenetic reconstruction
- Published
- Accepted
- Received
- Academic Editor
- Victoria Sosa
- Subject Areas
- Biodiversity, Plant Science, Taxonomy
- Keywords
- Argentina, Field study, Morphology, New species, Orchidaceae, Phylogenetic analyses, Pachygenium
- Copyright
- © 2022 M. Martin et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Pachygenium laurense (Orchidaceae, Spiranthinae), a new orchid species from Argentina—morphological evidence and phylogenetic reconstruction. PeerJ 10:e13433 https://doi.org/10.7717/peerj.13433
Abstract
Background
Pachygenium embraces a group of terrestrial species formerly placed in Pelexia sensu lato. The genus currently comprises some 60 species, most of which are known from the southern parts of Brazil and Paraguay, with few species distributed in the Andean countries—only four species have been recorded from Argentina so far. In Jujuy Province, Argentina a new species of Pachygenium was found during our fieldwork. The aim of this article was to provide morphological and molecular evidence for its membership in this genus.
Methods
Materials from specimens were collected in the field and examined by classical taxonomic and molecular biological techniques, e.g., PCR and sequencing DNA. Phylogenetic reconstruction was performed by maximum-likelihood and Bayesian inference.
Results
Pachygenium laurense from Argentina is described and illustrated based on morphological evidence and its taxonomic position was confirmed by phylogenetic analyses. A new combination for Pachygenium gutturosa is also proposed. A key for identification is provided for the Pachygenium species occurring in Argentina.
Conclusion
Pachygenium laurense is the fifth species of the genus recorded from Argentina.
Introduction
The name Pachygenium was established by Schlechter (1920), who distinguished it as one of the five sections of the genus Pelexia Poit. ex Lindley (Orchidaceae, Spiranthinae). Based mainly on the spur structure, Schlechter recognized, aside from Pachygenium, the following sections: Cogniauxiocharis, P otosia, Eupelexia, and Centropelexia. The genus Pelexia, in its broad circumscription, is difficult to define in the terms of morphology. As a result, the taxonomic status of some of the aforementioned sections has changed. Cogniauxiocharis was incorporated in Pteroglossa Schltr. (Garay, 1982), and Potosia was erected to the generic level by Tamayo & Szlachetko (Mytnik, 2003); Szlachetko, González & Rutkowski (2001) proposed Pachygenium to be assigned the rank of genus, which was subsequently supported by molecular analyses (Salazar et al., 2018).
Pachygenium embraces a group of terrestrial species which are rather easily distinguished from Pelexia s.s. by several characters. In all species of this genus the leaves have narrow, cuneate blades gradually transforming into indistinct petioles (vs. blade ovate to cordate with cordate to truncate base, distinctly diverse from the very narrow petiole). The spur is saccate and short, usually reaching the middle of the ovary (vs. spur usually cylindrical, acute at apex, often equal in length to or even longer than pedicel and ovary). The lateral sepals of Pachygenium are deeply saccate at the base, a feature lacking in Pelexia s.s. The strongly S-curved basal part of the lip of Pachygenium effects that the auricles are perpendicular to the ovary axis. Unlike this, the auricles and basal part of the lip of Pelexia s.s. are parallel to the ovary. A short and massive gynostemium with short and relatively wide rostellum are characteristics of Pachygenium, and different in Pelexia s.s., where the gynostemium is elongate, slender, with a narrow and linear rostellum. The viscidium in the former is transversely elliptic and solid, and in the latter small and delicate.
As thus defined, Pachygenium currently counts about 60 species (Mytnik-Ejsmont, Szlachetko & Górniak, 2010). The greatest diversity of the genus occurs in the southeastern and southern regions of Brazil, with a secondary center of diversity in Paraguay and only few species reported from Uruguay and the Andean countries.
According to the Flora of Argentina (http://www.floraargentina.edu.ar) the genus Pelexia s.l. is represented in that country by eight species, of which four are classified as belonging to Pachygenium: P. bonariense (Lindl.) Szlach., R. González & Rutk., P. ekmanii (Kraenzl.) Szlach., R. González & Rutk., P. ovatifolium (M.N. Correa) Szlach., R. González & Rutk., and P. paludosum (M.N. Correa) Szlach., R. González & Rutk. The fifth species, P. saltense (Griseb.) Szlach., R. González & Rutk., is often treated as a synonym of P. bonariense (Szlachetko, González & Rutkowski, 2001).
Our field studies on the biodiversity of plants in Argentina resulted in the discovery of a new species of orchid. The analyses based on morphological features indicate its membership in the genus Pachygenium. Moreover, phylogenetic reconstruction was performed using nuclear and plastid markers that also support this result. Its morphology does not match the characters of any other known representative of the genus. The aim of this study was to describe this species as new and to provide key for identification to all species of Pachygenium from Argentina.
Materials & Methods
Morphological study
All materials collected in the field were examined according to standard procedures. Each studied sample was photographed, and the flowers were placed in liquid. The form, size, and surface of leaves, tubular sheaths enveloping the scape, as well as details of the inflorescences (including form of the floral bracts and ovaries) were examined. The perianth segments were scrutinized and measured under a stereoscopic microscope. The obtained data were compared with original diagnoses and illustrations of representatives of Pelexia and Pachygenium from Argentina and adjacent countries. We examined over 1000 specimens of these genera stored in AAU, AMES, AMO, B, BA, BIGU, BM, C, COAH, COL, E, F, G, GOET, K, L, M, MO, P, S, SEL, SP, U, US, W, WRSL, WU, and Z.
Information on the occurrence and habitats of the new species was verified during field studies conducted in Argentina in 2020 and 2021. All field experiments complied with provincial, state, and national laws. The Dirección Provincial de Biodiversidad de Jujuy–Secretaría de Gestión Ambiental (Jujuy, Argentina) provided permission to conduct the research under permit number 171/2015-DPB.
“The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants (ICN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. In addition, new names contained in this work which have been issued with identifiers by IPNI will eventually be made available to the Global Names Index. The IPNI LSIDs can be resolved and the associated information viewed through any standard web browser by appending the LSID contained in this publication to the prefix “http://ipni.org/”. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central SCIE, and CLOCKSS”.
Molecular analyses
For phylogenetic reconstruction we applied 84 sequences of the ITS region, 90 of the trnL-trnF marker, and 75 of the matK gene representing taxa from seven genera: Cyclopogon, Veyretia, Glohisarcon, Sarcoglottis, Pelexia, Brachystele, and Pachygenium. In selecting the samples for ours analyses, we relied on the results obtained by Salazar et al. (2018). To determine the phylogenetic relations for the new taxon, we used species that grouped together with Pachygenium and Pelexia. Salazar et al. (2018) showed that representatives of Cyclopogon and Veyretia appear as a sister group, therefore we also used these as samples. Cyclopogon obliquus was selected to root the trees.
Most of the sequences used in this article were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/). A list of the taxa with their accession numbers is included in Appendix 1. Samples (leaf fragments) of Pachygenium laurense used for molecular studies were collected in the field by CM. The exact localities with numbers for names and collector are presented in Table 1. GenBank accession numbers for the sequences for these samples are listed in Table 2.
| Taxon | Locality | Date | Collector | No |
|---|---|---|---|---|
| Pachygenium laurense 1 | ARGENTINA. Jujuy, Dept. San Pedro. San Juan de Dios, Finca Las Lauras [S S24°33′49.43″; W64°39′17.77″; 941msl] | 20 Feb 2021 | Claudia M. Martín | 2873 |
| Pachygenium laurense 2 | ARGENTINA. Jujuy, Dept. San Pedro. San Juan de Dios, Finca Las Lauras [S 24°33′48.13″; W64°39′23.63″; 916msl] | 20 Feb 2021 | Claudia M. Martín | 2874 |
| Pachygenium laurense 3A | ARGENTINA. Jujuy, Dept. San Pedro. San Juan de Dios, Finca Las Lauras [S 24°33′39″; W 64°39′26,9″; 906msl] | 21 Feb 2021 | Claudia M. Martín | 2875 |
| Pachygenium laurense 3B | ARGENTINA. Jujuy, Dept. San Pedro. San Juan de Dios, Finca Las Lauras [S 24°33′39″; W 64°39′26,9″; 906msl] | 21 Feb 2021 | Claudia M. Martín | 2876 |
| Taxon | No GenBank of ITS | No GenBank of matK gene | No GenBank of trnL-trnF |
|---|---|---|---|
| Pachygenium laurense 1 | OL619328 | OL694863 | OL631601 |
| Pachygenium laurense 2 | OL619327 | OL694861 | OL631600 |
| Pachygenium laurense 3A | OL619330 | —– | OL631599 |
| Pachygenium laurense 3B | OL619329 | OL694862 | OL631602 |
DNA Isolation. Total genomic DNA was extracted from 20–100 mg of dried leaves (Chase & Hills, 1991) using the DNA Sherlock AX Kit (A&A Biotechnology, Poland) following the manufacture’s protocol. The pellets of DNA were suspended in 50 µl of TE buffer.
Amplification and sequencing. The PCRs and sequencing reactions were performed for three markers, two plastid (matK and trnL-trnF) and one nuclear (ITS1+5.8S+ITS2). In both, the same pairs of primers for each marker were used: for the ITS region, 101F and 102R primers (Douzery et al., 1999) while for the plastid region trnL-F containing the trnL intron and trnL-trnF intergenic spacer using primers trnL-c and trnL-f as described by Taberlet et al. (1991), and for the part of the matK gene, primers 19F (Molvray, Kores & Chase, 2000) and 1326R (Cuénoud et al., 2002).
The total volume of sample for amplification was 25 µl containing 1 µl template DNA (∼10–100 ng), 0.5 µl of 10 µM of each primer, 11 µl MyTaq HS DNA Polymerase Mix (BIOLINE Ltd., UK), and water. Parameters for the PCR reaction for nrITS (ITS1+5.8S+ITS2) were: 94 °C, 4 min; 30 × (94 °C, 45 s; 52 °C, 45 s; 72 °C, 1 min), and 72 °C, 7 min. For plastid markers, conditions for the matK gene were: 95 °C, 3 min; 33 × (94 °C, 45 s; 52 °C, 45 s, 72 °C, 90 s); 72 °C, 7 min, and for the trnL-F region: 94 °C, 3 min; 35 × (94 °C, 60 s; 55 °C, 60 s, 72 °C, 120 s); 72 °C, 7 min. All products of the PCR reaction were tested using electrophoresis in 1% agarose gel with at 110 V for 25 min. The Clean-Up Concentrator Kit (A&A Biotechnology, Poland) was used to clean the PCR products following the manufacturer’s protocol, and eluted with 30 µl of nuclease-free water.
Purified PCR products were sequenced by Macrogen (Seoul, South Korea - http://dna.macrogen.com/eng/), using the same primers mentioned above. DNA sequence chromatograms were examined/edited in FinchTV (https://finchtv.software.informer.com/1.4/).
Phylogenetic reconstruction. First, multiple sequence files for single markers were aligned with SeaView (Galtier, Gouy & Gautier, 1996). We used “align” option according the MUSCLE algorithm (Edgar, 2004). All alignments were inspected, possibly corrected and trimmed to equal length with SeaView (Galtier, Gouy & Gautier, 1996). Maximum-likelihood (ML) and Bayesian inference (BI) were used for phylogenetic reconstruction to test for possible topological incongruence. Best fit substitution models were obtained with the jModelTest 2 (Darriba et al., 2012) (GTR+G+I for the matK matrix and GTR+G for the datasets ITS and trnL-trnF).
The ML/Thorough bootstrap workflow analysis was performed using RAxML-HPC2 (Stamatakis, 2014) by searching for the best-scoring ML tree under the previously calculated nucleotide substitution model. The branch support values (BS) were calculated with RaxML by halting bootstrapping automatically under the autoMRE criterion.
Bayesian inference was performed using MrBayes v. 3.2.7a (Ronquist et al., 2012). Two independent runs of four Markov-chain Monte Carlo (MCMC) chains (one heated and three cold) were started from different random trees to ensure that individual runs had converged to the same result. We used 2 million generations for ITS and matK matrices, and 3 million for the trnL-trnF dataset per run with sampling every 100 generations. Convergence was assessed using the average standard deviation of split frequencies below 0.01 and the effective sample size (ESS) was checked in the Tracer v. 1.7.1 (Rambaut et al., 2018). Thereafter we discarded the initial 25% of the sampled generations of each chain as burn-in. Saved trees were summarized in a majority rule consensus tree and were edited with FigTree v.1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) and Inkscape (https://inkscape.org/release/inkscape-1.0.2/). The nodal confidence was assessed by posterior probabilities (PP), which were considered strongly supported when equal to or higher than 0.95 (Huelsenbeck & Ronquist, 2001).
Results
The results of the maximum-likelihood and Bayesian inference gave similar results. Thus, we present and discuss the 50% majority-rule consensus tree only. The clades that have low support for bootstrap values in the trees of the ML analyses also have low posterior probability values in trees resulting from Bayesian inference. The topology of trees obtained for the matK and the trnL-trnF regions are similar. Therefore, we decided to perform combined analyses for these markers. We also made a combined analysis for all used fragments of DNA. In this article we present the trees from the ITS region (Fig. 1) and combined matrices (Figs. 2–3). All other obtained trees are available from the corresponding author. Table 3 lists the statistical data.
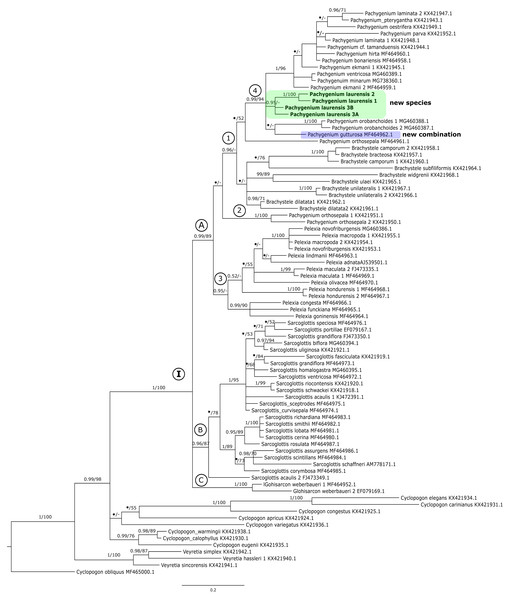
Figure 1: The 50% majority-rule consensus tree obtained for the ITS marker showing the position of Pachygenium laurense.
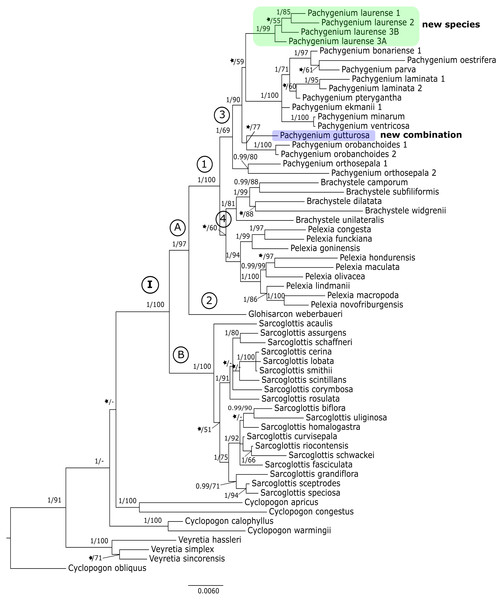
Posterior probability (PP) and bootstrap support (BS) values are indicated above the branches; BS values of <50% as –, PP < 0.95 as an asterisk.Figure 2: The 50% majority-rule consensus tree obtained for the combined matrix of plastid regions showing the position of Pachygenium laurense.
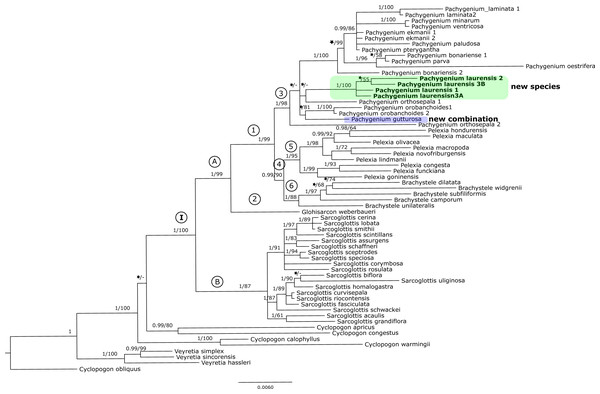
Posterior probability (PP) and bootstrap support (BS) values are indicated above the branches; BS values of <50% as –, PP < 0.95 as an asterisk.Figure 3: The 50% majority-rule consensus tree obtained for the combined matrix of nuclear and plastid regions showing the position of Pachygenium laurense.
Posterior probability (PP) and bootstrap support (BS) values are indicated above the branches; BS values of < 50% as –, PP < 0.95 as an asterisk.ITS matrix
In the ITS tree representatives of Cyclopogon and Veyretia were placed at its base (Fig. 1). One of species of Cyclopogon (C. obliquus) served as an outgroup. The genus Veyretia is represented by three species in our analyses and all of them are included in one strongly supported group at the base of the tree. The examined species of Cyclopogon did not form a coherent clade and were recovered in a polytomy with clade I (Fig. 1). The main clade I of this tree embraces representatives of Glottisarcon, Sarcoglottis, Pelexia, Brachystele, and Pachygenium. All these taxa appear to be closely related as evidenced by strong support (PP = 1, BS = 100). In clade I we can recognize three smaller groups (clades A-C, Fig. 1). The first one (clade A) with strong support, both posterior probability and bootstrap value (PP = 0.99, BS = 89) includes species of Pelexia, Brachystele, and Pachygenium. Representatives of Pelexia (subclade 3, Fig. 1) formed a consistent group with strong support but only by posterior probability (PP = 0.95). The results of the ML analysis did not yield any support for this clade. We observed a similar situation in subclade 1 that embraces taxa of Pachygenium and Brachystele. It is worth noting that samples of Pachygenium orthosepala are not gathered in one clade. Two of them (KX421950, KX421951) were placed together (Fig. 1, subclade 2). The third sample of P. orthosepala (MF464961) is included in subclade 1 with other Pachygenium and Brachystele species. The results of the BI analysis showed that species of Brachystele and Pachygenium, expect P. orthosepala 1 and 2, are grouped together with strong support (subclade 1, PP = 0.96, Fig. 1). However, this is not supported by the results of the ML analysis, and it is the only difference in the results in these methods. Pachygenium laurense in our study was represented by four samples which form a strongly supported group (PP = 0.95) and it is placed with other representatives of Pachygenium (subclade 4, Fig. 1).
Clade B embraces taxa of Sarcoglottis, while the samples of Glottisarcon weberbaueri formed clade C (Fig. 1). Both these groups are strongly supported (PP = 0.96, BS = 87; PP = 1, BS = 100) and closely related to representatives of clade A.
Combined matrices (matK + trnL-trnF and ITS + plastid markers)
The trees obtained for the combined matrices showed similar results as for the nuclear marker. Again, Cyclopogon obliquus is an outgroup, whereas species of Veyretia and the other sampled species of Cyclopogon were placed at the base of this phylogram. The main group of this tree (clade I, Figs. 2–3) embraces representatives of Glottisarcon, Sarcoglottis, Pelexia, Brachystele, and Pachygenium. Clade I was strongly supported both by posterior probability and bootstrap value (PP = 1, BS = 100) and it is divided into two smaller groups. The first, clade A, (Figs. 2–3) indicated a sister relationship between the taxa of Pelexia, Brachystele, and Pachygenium (clade 1) and Glottisarcon weberbueri. The members of Pachygenium, including the new species, formed the monophyletic clade 3 (Figs. 2–3) with strong support, both by posterior probability and bootstrap values. P. laurense is represented by four samples, all of which grouping together with one clade of high bootstrap support (BS = 100) and posterior probability (PP = 1).
| Matric/Data | No of taxa | Total characters | Constant Characters | Informative characters |
|---|---|---|---|---|
| ITS1-5.8S-ITS2 | 84 | 823 | 542 | 163 |
| part of matK gene | 75 | 1881 | 1399 | 186 |
| trnL-trnF region | 90 | 1440 | 1052 | 182 |
| combined of plastid markers | 63 | 3247 | 2567 | 272 |
| combined of all markers | 60 | 4034 | 3132 | 392 |
The species of Pelexia and Brachystele (clade 4) (Figs. 2–3) run as two consistent clades, sisters to each other, and both are strongly supported (PP = 1, BS = 98; PP = 0.99, BS = 88). The second one, clade B, includes species of Sarcoglottis (Figs. 2–3).
Discussion
The new species described in this article is an example of an unusual combination of various characters. While in flowering, Pachygenium laurense brings to mind Porphyrostachys parviflora (C. Schweinf.) Garay from Peru, especially with its leafless stem and densely many-flowered inflorescences. The ecallose, shortly clawed lip resembles that of Eltroplectris Raf., but the relatively slender elongate gynostemium appears to be more similar to that of Pelexia s.s. The narrow leaf blade gradually transiting into the petiole, and the saccate spur suggests a relationship with Pachygenium.
Our analyses of molecular markers have been able to help to clarify the taxonomic position of this odd species. The phylogenetic trees obtained for the single markers (i.e., ITS, matK, and trnL-trnF) (Fig. 1) and the combined matrices (Figs. 2–3) reveal that this taxon is accommodated in the Pachygenium clade. There are several characters, however, that are unique for this species and unknown for other species of Pachygenium as described to date. There are some species of Pachygenium growing in drier areas which can have either well-developed leaves or leaves withering in flowering. At the best of our knowledge, P. laurense appears to be the only species of the genus in South America which is totally leafless at anthesis. Interestingly, Pelexia gutturosa Rchb.f. from Mesoamerica, has very similar leafless habit and general flower architecture. Furthermore, P. gutturosa also grouped with the Pachygenium clade in our analysis (clade 4, Fig. 1 and clade 3 Figs. 2–3). Therefore, we decided to transfer this species to the genus Pachygenium as well.
The other unique features of the new species are: very characteristic arrangements of the flowers in the inflorescence, ecallose lip, undivided into epichile and hypochile and narrow gynostemium. In our opinion these features afford the plants to be assigned status of species and we describe it below.
Taxonomic treatment
Type: ARGENTINA. Jujuy: Dep. San Pedro, San Juan de Dios, Estancia Las Lauras, S 24°33′39″; W 64°39′26,9″, 906 msl., 03-Sep-2020, C.M. Martín 2814 (holotype: JUA [JUA2814CMM]!; [UGDA-DLSz! drawing, photos]).
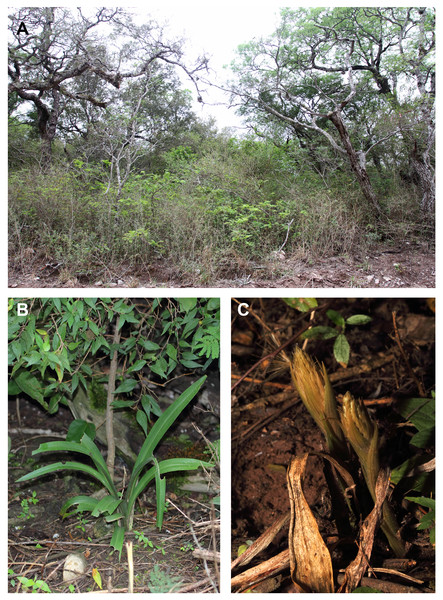
Figure 4: Pachygenium laurense in situ.
(A) Habitat. (B) Leaves. (C) Inflorescence buds (photos by Claudia M. Martín).Figure 5: Floral structures of Pachygenium laurense.
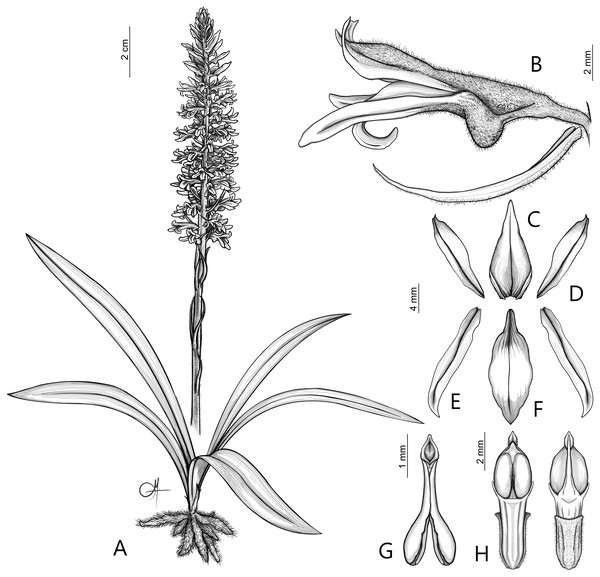
(A) (E) Inflorescence, lateral view (B) Inflorescence, top view. (C) Flower, front view. (D) Flower, lateral view (photos by Claudia M. Martín).Figure 6: Drawing of Pachygenium laurense.
(A) Habit. (B) Flower, lateral view. (C) Dorsal sepal. (D) Lateral sepals. (E) Petal. (F) Lip. (G) Pollinarium. (H) Gynostemium, front and back view (drawn by Adriana M. Morales).Diagnosis: Species leafless at anthesis, with very characteristic arrangements of the flowers in inflorescence, ecallose, undivided lip, and narrow, slender gynostemium.
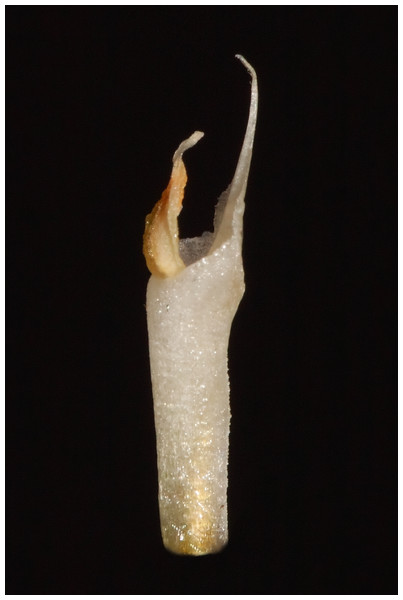
Description: Plants terrestrial. Roots fasciculate, tuberous, fleshy, thin, spindle to cylindrical, hairy. Leaves 3–4(6), basal, rosulate, 22–24 × 1.5–2.8 cm, sessile, somewhat fleshy, linear-oblanceolate, acute, absent when flowering. Inflorescence erect, up 20 cm long, densely many-flowered; peduncle reddish, glandulous pubescent; sheaths 6–11, (2.5) 4 × 1 cm, the lower ones larger; rachis 16–18 cm, glandulous pubescent; floral bracts 1.8 × 0.3 cm, narrowly lanceolate, acuminate, reddish, glandulous pubescent along midvein. Flowers about 50, resupinate, rather small, cream-whitish-green in general appearance, densely glandular. Pedicellate ovary ca 0.5–0.7 cm long, densely glandular, greenish. Dorsal sepal 10 × 5 mm, ovate-lanceolate, acute, concave in the lower half, white with greenish central line on inner surface, greenish outside, densely glandular at the base, sparsely glandular above. Lateral sepals 12–14 × 2–2.5 mm, linear-lanceolate above saccate base, apically falcate, acute, white with a reddish-brown central line pubescent, decurrent on the column foot forming a chin shorter than the ovary, densely glandular at the base, sparsely above. Petals 10 × 2.2 mm, oblanceolate-ligulate above narrow base, somewhat oblique, acute, white with a greenish central line, connecting with the dorsal sepal, pubescent at the base. Lip 12 × four mm, slightly fleshy, with a narrow basal claw, elliptic-lanceolate, acute, margins somewhat wavy, ecallose, ±canaliculated in the lower half, recurved and somewhat flattened above, white with a reddish-brown central line. Gynostemium seven mm long, slender, erect, semi-fusiform, papillate on the ventral surface (Fig. 7). The anther 2 mm long, elliptic with acute apex. Pollinarium 2.5–3 mm long, basal half very narrow, slender, apical part somewhat swollen, spread. Stigma bilobed, both lobes confluent. Rostellum two mm long, terminal, linear, somewhat upcurved. Viscidium 0.5 mm long, produced on the upper surface of the rostellum, triangular-ovate in outline, acute. Clinandrium narrow.
Figure 7: The gynostemium of Pachygenium laurense (photo by Claudia M. Martín).
Etymology: The name of the species refers to the place where it was collected for the first time (Estancia Las Lauras).
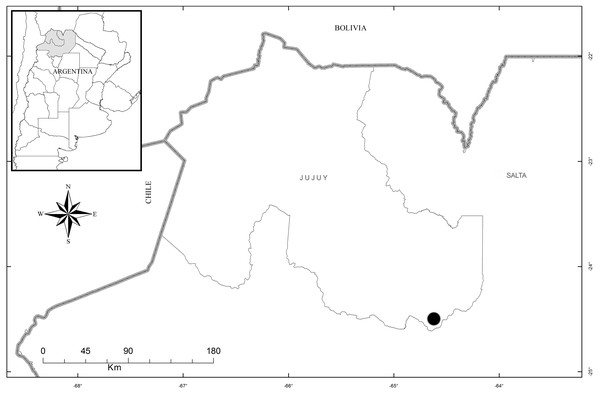
Distribution, habitat, and ecology: The new species is restricted in distribution to the Estancia Las Lauras in San Pedro (Jujuy, Argentina) (S 24°34′16.1″; W 64°39′03.7″) (Fig. 8). The altitude of this locality is 995 m a. s. l., with a mean annual temperature from 17.7–20.2 °C, and a mean annual precipitation of 431–737 mm (Entrocassi et al., 2014). The area belongs to the phytogeographic district of the Bosque Chaqueño Occidental (Oyarzabal et al., 2018). Pachygenium laurense grows in the understory of a xerophilous and deciduous forest of Schinopsis lorentzii (Griseb.) Engl., Alvaradoa subovata Cronquist, Libidibia paraguariensis (D. Parodi) G.P. Lewis, Gochnatia palosanto Cabrera, Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart, Ceiba chodatii (Hassl.) Ravenna and Athyana weinmanniifolia (Griseb.) Radlk. Flowering occurs in the dry season, from late August to mid-September, when the plants are completely leafless. The leaves develop at the end of the rainy season.
Figure 8: Map of the distribution of Pachygenium laurense (circle) (prepared by Claudia M. Martín).
Information about the population: During our studies we found less than 15 individuals, most of were growing close to each other. Thus, we considered that the individuals are aggregated and scarce.
Conservation status: Pachygenium laurense has only been recorded for the type locality, in an area smaller than 1 km2. In addition, the surrounding Chaco forests, where it is probable to find more populations of P. laurense, are being replaced by crops or livestock. For these reasons, the new species is classified as Critically Endangered CR [criteria C2a(i)] according to the World Conservation Union Red List Categories and Criteria (IUCN, 2019).
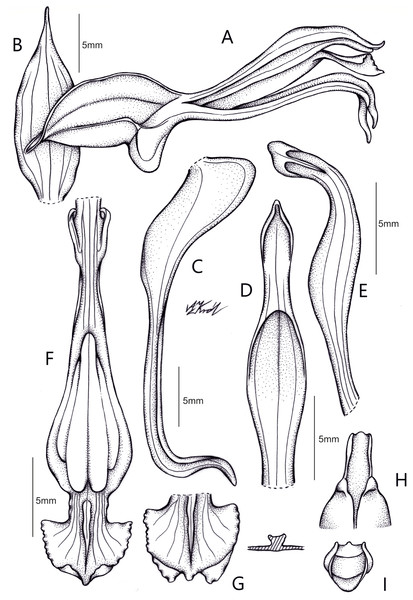
Pachygenium gutturosa (Rchb.f.) Szlach., Dudek & C.M. Martín, comb. nov. (Fig. 9)
Figure 9: Drawing of Pachygenium gutturosa com. nov. from type material H. Wendland 427 (W-R35230).
(A) Flower, side view. (B) Floral bract. (C) Lateral sepal. (D) Dorsal sepal. (E) Petal. (F) Lip. (G) Apical part of lip. (H) Gynostemium apex, bottom view. (I) Viscidium (drawn by Anna Król).Basionym: Spiranthes gutturosa Rchb.f., Beitr. Orchid.-K. C. Amer. 67. 1866; Type: EL SALVADOR, St. Vincent, 13 Feb 1857, H. Wendland 427 (holotype: W-R!). ≡ Sarcoglottis gutturosa (Rchb.f.) Ames ex Donn. Sm., Enum. Pl. Guatem. 7: 49. 1905. ≡ Pelexia gutturosa (Rchb.f.) Garay, Bot. Mus. Leafl. 28(4): 344. 1980-1982.
Key to the species of Pachygenium in Argentina
1. Plant leafless at anthesis, lip entire, undivided, elliptic-lanceolate, acute, without basal auricles ……………………………………………………………………………... P. laurense
1* Plant leafy at anthesis, lip ±constricted, basally auriculated, lamina not above …………………………………………………………………………………………2
2. Leaves oblong-obovate to elliptic-obovate, 5–6 cm wide ……………………... P. ovatifolium
2* Leaves not as above …………………………………………………………………... 3
3. Lip lamina narrow, strip-like in a basal part, much expanded above, ±ovate ………………………………………………………………………………P. paludosum
3* Lip not as above ……………………………………………………………………….... 4
4. Petals constricted in the apical 5th or 6th, apex obliquely rhombic, acute ……….. P. saltense
4* Petals oblong ligulate, obtuse ……………………………………………………………5
5. Lip distinctly constricted, hypochile oblong triangular to ovate ………………. P. bonariense
5* Lip somewhat constricted, hypochile oblong ……………………………………. P. ekmanii