Iris sanguinea is conspecific with I. sibirica (Iridaceae) according to morphology and plastid DNA sequence data
- Published
- Accepted
- Received
- Academic Editor
- Gabriele Casazza
- Subject Areas
- Molecular Biology, Plant Science, Taxonomy
- Keywords
- Chloroplast DNA, Iris subser. Sibiricae, Molecular phylogeny, Morphology, Nomenclature, Taxonomy
- Copyright
- © 2020 Boltenkov et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Iris sanguinea is conspecific with I. sibirica (Iridaceae) according to morphology and plastid DNA sequence data. PeerJ 8:e10088 https://doi.org/10.7717/peerj.10088
Abstract
A taxonomic revision of Iris subser. Sibiricae is provided based on morphological and molecular analyses and the study of protologues and original material. Two to three species have been recognized in this subseries by botanists. To address the question of species delimitations and relationships within this group, we analyzed four non-coding regions of plastid DNA (trnS–trnG, trnL–trnF, rps4–trnSGGA, and psbA–trnH) for samples from 26 localities across the distribution ranges of two currently recognized species, I. sanguinea and I. sibirica. Variance analysis, based on nine characters, revealed no separation between taxa. Moreover, no morphological character could be used to define clear boundaries between taxa. Our results strongly support that I. subser. Sibiricae is monotypic and comprises only I. sibirica, instead of two or three species. Iris sibirica is morphologically variable and one of the most widespread Eurasian species of Iridaceae. Previously accepted taxa, I. sanguinea and I. typhifolia, are synonymised with I. sibirica and also two names, I. orientalis and I. sibirica var. haematophylla, which are typified here, are placed in the synonymy of I. sibirica. Information on the distribution of I. sibirica and the main features used to distinguish between I. sibirica and I. subser. Chrysographes species are provided.
Introduction
Iris L. is the largest, most widespread in Iridaceae distributed mainly in the temperate zones of the Northern Hemisphere. Iris is a taxonomically difficult genus. Its generic limits are controversial, and recent data seem to favour a much narrower circumscription (Crespo, Martínez-Azorín & Mavrodiev, 2015). However, the infrageneric composition and circumscription of Iris is questionable (Boltenkov et al., 2018). Therefore, we believe that additional studies are needed, and thus, a conservative taxonomy is here applied (Mathew, 1989; Wilson, 2009).
While revising I. sect. Limniris Tausch, we find that the taxonomy of I. ser. Sibiricae (Diels) G.H.M.Lawr. still remains unclear. Plants of this Eurasian group are rhizomatous herbs distinguished from all the other Iris species, except I. clarkei Baker ex Hook.f., by having a hollow flowering stem. The infrageneric taxon Sibiricae was first described by Diels (1930) as a subsection, including eight species with a short tube, a triangular elongated stigma, narrow grassy leaves, in cross-section triangular capsules, and disc-shaped or nearly cubical seeds. These species were later subdivided into two groups on account of their chromosome numbers (Simonet, 1934), morphology and geographical distribution (Grey-Wilson, 1971; Lenz, 1976). The autonymic subseries of I. ser. Sibiricae includes well-known garden ornamentals, with 2n = 28 chromosomes (Löve, 1975; Probatova, 2006), that hybridise easily both in the garden and in the wild (McEwen & McGarvey, 1978; Grey-Wilson, 2012), and are known to horticulturists under the name of Siberian irises. The other group, I. subser. Chrysographes (Simonet) L.W.Lenz, comprises species with 2n = 40 chromosomes, and are known to horticulturists as Sino-Siberian irises (Waddick & Zhao, 1992). These latter irises are native to southwestern China (mainly Yunnan and Sichuan provinces) and eastern Himalayas, and occur at high elevations (Zhao, Noltie & Mathew, 2000; Grey-Wilson, 2012). The distinctness of these two groups within I. ser. Sibiricae was also supported by previous molecular studies (Tillie, Chase & Hall, 2000; Wheeler & Wilson, 2014; Crespo, Martínez-Azorín & Mavrodiev, 2015).
The species’ circumscription of Siberian irises differed among later botanists, who distinguished either two (McEwen & McGarvey, 1978; Mathew, 1989; Doronkin, 2012) or three species (Rodionenko, 2007; Zhao, Noltie & Mathew, 2000; Grey-Wilson, 2012; Crespo, Martínez-Azorín & Mavrodiev, 2015) in this group: I. sanguinea Hornem., I. sibirica L., and I. typhifolia Kitag.
Iris sibirica was first described by Linnaeus (1753) from Austria, Switzerland, and Siberia. Authors from the end of the 19th century (e.g., Baker, 1877; Hooker, 1899) believed that I. sibirica is one of the most widespread species of Iridaceae in Eurasia, extending from Central Europe to Japan. Therefore, I. sibirica has been considered as a single species including several varieties (Regel, 1867; Baker, 1877; Maximowicz, 1880; Komarov, 1901; Dykes, 1910).
Iris sanguinea was formally described by Hornemann (1813) based on cultivated plants from the Botanical Garden of Copenhagen, Denmark. Subsequently, I. sanguinea was reduced to a variety of I. sibirica, i.e., I. sibirica var. sanguinea (Hornem.) Ker Gawl., characterized by having young leaves often red-tinged at base. Some authors (e.g., Spach, 1846; Ledebour, 1852) cited this variety under the name I. sibirica var. haematophylla Besser. At the same time, plants from the eastern regions of Eurasia were indicated under the names I. sibirica var. sanguinea, I. sibirica var. haematophylla, and I. sibirica var. orientalis (Schrank) Baker. Koidzumi (1926) re-established I. sanguinea, indicating a distribution range including Japan, Dauria (currently Transbaikal region), and the Amur River basin. As a result, this taxon was accepted as being native to temperate regions of East Asia by all subsequent authors (e.g., Pavlova, 1987; Mathew, 1989), or it was cited under the illegitimate name I. orientalis Thunb. (e.g., Dykes, 1912; Diels, 1930; Fedtschenko, 1935; Lawrence, 1953).
It has been stated that I. sanguinea and I. sibirica are morphologically barely distinguishable (Komarov, 1901; Dykes, 1912; Grubov, 1977), and their identification is mostly based on the inflorescence structure (McEwen & McGarvey, 1978; Mathew, 1989; Grey-Wilson, 2012). In the I. subser. Sibiricae species, the inflorescence is cymose and formed by the terminal head of flowers and one or two lateral heads (Szöllösi et al., 2011; Skrypec & Odintsova, 2017). According to several authors (Dykes, 1912; Mathew, 1989; McEwen & McGarvey, 1978; Grey-Wilson, 2012), the typical I. sanguinea individuals generally produce stem bearing the terminal head, while I. sibirica individuals produce a stem with terminal and lateral heads. According to Skrypec & Odintsova (2017), I. sibirica inflorescences have a high morphological variability in the number of flowers, their position, and the flowering order. Other studies (Dénes, Juhász & Salamon-Albert, 2008; Szöllösi et al., 2011) indicated that the inflorescence features in I. sibirica vary through years and depend on climatic parameters.
Iris typhifolia, the third species recognized in I. subser. Sibiricae by some authors, was described by Kitagawa (1934) as a Chinese endemic on the basis of one specimen. This specimen was collected in the northern part of the Beiling District (currently Shenyang City, Liaoning Province) and originally identified as I. sibirica (see Taxonomic treatment below). Kitagawa (1934) specified that I. typhifolia is distinct from other irises by having slender twisted leaves. Waddick & Zhao (1992) suggested that I. typhifolia differs from I. sanguinea by its narrow leaves, generally about 0.2 cm wide. Nevertheless, Grey-Wilson (2012) noticed that the cultivated plants of I. typhifolia appeared to differ from the original description (0.15–0.22 cm wide) in having broader leaves.
Fedtschenko (1949) noticed that the eastern boundary of the distribution range of I. sibirica is the Sayan Mountains in southern Siberia (Russia). According to recent studies (McEwen & McGarvey, 1978; Mathew, 1989; Galanin, 2009; Grey-Wilson, 2012), the identification of I. sanguinea and I. sibirica has often been based on their geographical origin: I. sibirica has been considered to be distributed in Europe and Western Siberia, while I. sanguinea has been considered to occur in East Asia, eastward Lake Baikal (also see Global Biodiversity Information Facility, 2020). Iris typhifolia has been reported from the same Chinese provinces where I. sanguinea has also been reported (Zhao, Noltie & Mathew, 2000). Furthermore, it has recently been found that the typical plants of I. typhifolia described by Kitagawa (1934) are not found in the type locality, or in any other area in Liaoning Province whereas plants matching I. sanguinea have been recorded in this province (Zheng et al., 2017).
Integrative approaches combining morphological and molecular data obtained from plastid DNA (cpDNA) and nuclear ribosomal DNA (nrDNA) are widely used to distinguish taxa at different taxonomic ranks (Liu et al., 2012; Hu et al., 2015; Vicente, Alonso & Crespo, 2019). The nrDNA spacer regions provide information useful for phylogenetic reconstructions in plant systematics, though intraindividual nrDNA polymorphism can lead to erroneous or ambiguous results (Poczai & Hyvönen, 2010; Wilson, Padiernos & Sapir, 2016). Numerous studies have highlighted the great value of applying chloroplast DNA (cpDNA) sequence data for species delimitation in Iris (Tillie, Chase & Hall, 2000; Wilson, 2004; Wilson, 2009; Wilson, 2011; Wilson, 2017; Guo & Wilson, 2013). In previous studies, we investigated the taxonomy of I. sect. Psammiris (Spach) J.J.Taylor (Kozyrenko, Artyukova & Zhuravlev, 2009) and I. ser. Lacteae Doronkin (Boltenkov, Artyukova & Kozyrenko, 2016; Boltenkov et al., 2018) based on cpDNA analysis (Boltenkov, Artyukova & Kozyrenko, 2016; Boltenkov, 2018).
To reconstruct the relationships among species within I. subser. Sibiricae, we used morphological and molecular data. Our aims are: (1) to compare the morphological characters of living plants and herbarium specimens from the distribution range of I. ser. Sibiricae; (2) to resolve the phylogenetic relationships of the I. subser. Sibiricae species and of some other series of I. sect. Limniris using four cpDNA regions; (3) to ascertain whether the genetic relationships among I. sanguinea and I. sibirica are consistent with their current taxonomic classification as separate species; and (4) to compare the results of morphological and molecular studies in order to evaluate the number of species in I. subser. Sibiricae.
Materials & Methods
Morphological study
The I. subser. Sibiricae species descriptions available in literature (Krylov, 1929; Sergievskaya, 1972; Doronkin, 1987; Mathew, 1989; Pavlova, 1987; Zhao, Noltie & Mathew, 2000; Grey-Wilson, 2012) were examined. We evaluated the thirteen characters, which were selected from those typically used in the literature together with those considered relevant according to our personal observations (see Fig. 1). These characters are listed in detail in Table 1. The original material of I. sanguinea, I. sibirica, I. sibirica var. haematophylla, I. orientalis Thunb., and I. typhifolia (see Taxonomic treatment below) was studied. In total, 224 scaled specimens of well-developed plants in flowering or fruiting were measured (see Appendix S1). The specimens of I. sanguinea and I. sibirica have been checked through high resolution images available in virtual herbaria (herbarium codes according to Thiers, 2020): ABGI and VBGI (https://botsad.ru/herbarium/), E (https://data.rbge.org.uk/search/herbarium/), MHA and MW (https://plant.depo.msu.ru/), NS and NSK (http://herb.csbg.nsc.ru:8081/#fuzzy-label), PI, PRC and WU (https://herbarium.univie.ac.at/database/search.php). For I. typhifolia, 48 specimens were used: 27 specimens from the Chinese botanist Yu-Tang Zhao, an expert on Chinese Iridaceae (e.g., Waddick & Zhao, 1992; Zhao, Noltie & Mathew, 2000), collection at NENU, and also 21 specimens have been checked through images available in virtual Chinese herbaria ( http://www.cvh.ac.cn/). The morphological characters were measured using AxioVision 4.8 (Carl Zeiss, Germany), a freeware comprehensive images viewer.
Figure 1: Photos of living plants of Iris sibirica.
(A) Plant in habitat. (B) Inflorescence with the terminal head of two flowers. (C) Inflorescence with the terminal head and one lateral head. (D) Fruits. (E) Seeds.| No. | Character | Code | Remarks |
|---|---|---|---|
| 1 | Rosette leaf length (cm) | LL* | Measured from base to apex for the longest leaf in rosette |
| 2 | Rosette leaf width (cm) | LW* | Measured in its broadest place for the broadest leaf in rosette |
| 3 | Flowering stem height (cm) | SH* | Measured from base of stem to base of bracts |
| 4 | Inflorescence structure | IS* | Classified as inflorescence with terminal head (1) or with terminal and one lateral head (2) |
| 5 | Number of flowers | NF* | Flowers per stem |
| 6 | Number of cauline leaves | NC* | Leaves arising on the flowering stem |
| 7 | Cauline leaf length (cm) | CL* | Measured from base to apex for the upper leaf |
| 8 | Bract length (cm) | BL* | Measured from base to apex for the outer bract |
| 9 | Pedicel length (cm) | PL* | Measured for the first blooming flower in the terminal head |
| 10 | Flower colour | FC | According to literature data |
| 11 | Fruit length (cm) | FL | Obtained for all fruits from the specimens at fruiting |
| 12 | Fruit shape | FS | Obtained from the specimens at fruiting |
| 13 | Seed shape | SS | According to literature data |
Note.
Asterisk (*) indicates characters used in the variance analysis.
For morphometric data analysis, nine characters were used (see Table 1). In this study both parametric and non-parametric versions of a one-way variance analysis (ANOVA) were applied. The differences were considered significant at p-value < 0.05. As multiple statistical testing was performed, the calculated p-value was adjusted using the procedure proposed by Benjamini & Hochberg (1995). To test basic ANOVA assumptions Shapiro–Wilk test for normality and Levene’s test for equality of variances were used. The missing values in the original data table were imputed using corresponding median values according (Kuhn & Johnson, 2018). The Kruskal–Wallis test was chosen as a non-parametric ANOVA algorithm (Dodge, 2008). Principal components analysis (PCA) was used to visualize the distribution of the analyzed individuals over the space of morphometric characters. It was applied to all quantitative characteristics. Directions of principal components were described in the factor space by their highest correlation values (denoted by r) with original axes. Computations were performed by means of SciPy (Virtanen et al., 2020) and Scikit-Learn (Pedregosa et al., 2011) packages.
DNA extraction, amplification and sequencing
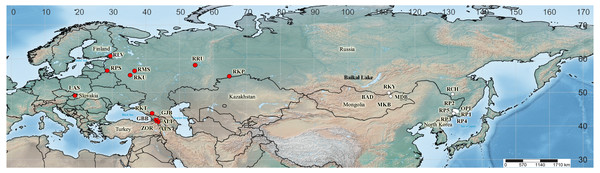
Sequences of four cpDNA regions were obtained for 44 specimens taken from wild populations, herbarium material and living collections. Among those, there were 20 from 13 localities in the I. sibirica distribution range, 22 from 11 localities in the I. sanguinea distribution range, and two plants were of unknown origin (Fig. 2). It was not possible to obtain samples from Japan and northeastern China, including Liaoning Province, where I. typhifolia was described from. Nevertheless, while searching GenBank for any sequences of four studied cpDNA regions of the I. subser. Sibiricae species, we found sequences of only either psbA–trnH or trnL–trnF for several accessions of I. sibirica and I. typhifolia, as well as I. sanguinea from Japan, northeastern China and the Republic of Korea (see Table S1). The sequences of four cpDNA regions from the complete chloroplast genome of I. sanguinea from the Republic of Korea (Lee et al., 2017) were included in the study. Our sampling also comprises representatives of three other series of I. sect. Limniris: (1) I. laevigata Fisch., I. ensata Thunb., and I. pseudacorus L. from I. ser. Laevigatae (Diels) G.H.M.Lawr.; (2) I. lactea Pall., I. oxypetala Bunge, and I. tibetica from I. ser. Lacteae; (3) I. uniflora Pall. ex Link from I. ser. Ruthenicae (Diels) G.H.M.Lawr. Iris dichotoma Pall. from I. subgen. Pardanthopsis (Hance) Baker was used as outgroup. The complete specimen list, including the sampling localities and the voucher information is given in Table 2.
Figure 2: Map showing the geographical origin of Iris subser. Sibiricae samples analyzed in the present study (created with https://www.simplemappr.net, CC 1.0).
Locality codes as in Table 2; cultivated plants (Sc1 and Sc2) are not mapped. Red circles –populations in the I. sibirica distribution range; white circles –populations in the I. sanguinea distribution range; black square –the locality of I. sanguinea from the Republic of Korea (Lee et al., 2017).| Code (N) | H | Locality, voucher | GenBank accession numberstrnH–psbA/rps4–trnS/trnS–trnG/trnL–trnF |
|---|---|---|---|
| I. ser. Sibiricae subser. Sibiricae | |||
| BAD (1) | H1 | Mongolia, Badgir, Dolgaleva s.n. (VBGI*) | LT627899/ LT628015/ LT628026/ LT628005 |
| MDB (1) | H1 | Mongolia, Dornod, Bayan-Uul, Gubanov 550 (MW) | LT978556/ LT981298/ LT984448/ LT984480 |
| MKB (1) | H1 | Mongolia, Khentii, Binder Somon, Galanin s.n. (VBGI) | LT978557/ LT981299/ LT984449/ LT984481 |
| ORL (3) | H2 | Russia, Primorsky Krai, Orlovka, Boltenkov s.n. (VBGI) | LT627900/ LT628016/ LT628027/ LT628006 |
| RCH (5) | H2 | Russia, Amur Oblast, Chingan State Nature Reserve, Kudrin s.n. (ARKH) | LT978531/ LT981273/ LT984423/ LT984456 |
| RP1 (1) | H1 | Russia, Primorsky Krai, Solovei Kluch, Boltenkov s.n. (VBGI) | LT978535/ LT981277/ LT984427/ LT984460 |
| RP2 (1) | H2 | Russia, Primorsky Krai, Khankaysky District, Il’inka, Pshennikova s.n. (VBGI) | LT978530/ LT981272/ LT984422/ LT984455 |
| RP3 (3) | H3 | Russia, Primorsky Krai, vicinity of Vladivostok, Kuritskaya s.n. (VBGI) | LT978534/ LT981276/ LT984426/ LT984459 |
| RP4 (1) | H3 | Russia, Primorsky Krai, Romanovka, Chubar s.n. (VBGI) | LT978533/ LT981275/ LT984425/ LT984458 |
| RP5 (2) | H3 | Russia, Primorsky Krai, Pokrovka, Denisova & Talovskaya s.n. (VBGI) | LT978532/ LT981274/ LT984424/ LT984457 |
| RKP (1) | H4 | Russia, Kurgan Oblast, Pritobolny District, Fedotova s.n. (NSK) | LT978536/ LT981278/ LT984428/ LT984461 |
| RKT (1) | H4 | Russia, Karachay-Cherkess Republic, Teberda, Shilnikov s.n. (cult.) | LT978529/ LT981271/ LT984421/ LT984454 |
| RKU (1) | H5 | Russia, Kaluga Oblast, Ugra National Park, Reshetnikova et al. s.n. (MHA) | LT978539/ LT981281/ LT984431/ LT984464 |
| RKY (3) | H1 | Russia, Zabaykalsky Krai, Mountain Steppe State Reserve, Roenko s.n. (VBGI) | LT978542/ LT981284/ LT984434/ LT984467 |
| RLV (1) | H6 | Russia, Leningrad Oblast, vicinity of Vyborg, Boltenkov s.n. (cult.) | LT978545/ LT981287/ LT984437/ LT984470 |
| RMS (1) | H7 | Russia, Moscow, Setun River valley, Nasimovitch & Shchukin s.n. (MHA) | LT978541/ LT981283/ LT984433/ LT984466 |
| RPS (8) | H5 | Russia, Pskov Oblast, Sebezhsky District, Konechnaya s.n. (LE) | LT978538/ LT981280/ LT984430/ LT984463 |
| RRU (1) | H4 | Russia, Udmurt Republic, Perevoznoye, Melnikov s.n. (LE) | LT978537/ LT981279/ LT984429/ LT984462 |
| ALS (1) | H4 | Armenia, Lori Province, Saratovka, Khanjyan & Tumanyan s.n. (ERE) | LT978528/ LT981270/ LT984420/ LT984453 |
| ALT (1) | H4 | Armenia, Lori Province, track from Dashtadem to Tashir, Tamanyan et al. 07-1189 (ERE) | LT978527/ LT981269/ LT984419/ LT984452 |
| GJP (1) | H4 | Georgia, Javakheti, between Aspara and Vladimirovka villages, Shvanova s.n. (LE) | LT978526/ LT981268/ LT984418/ LT984451 |
| GBB (1) | H8 | Georgia, Borjomi, Bakuriani Botanical Garden, Merello s.n. (cult.) | LT978543/ LT981285/ LT984435/ LT984468 |
| LAS (1) | H5 | Austria, Niederösterreich, Haltestelle Stillfried, Barta s.n. (ERE) | LT978544/ LT981286/ LT984436/ LT984469 |
| Sc1 (1) | H9 | United Kingdom, Cambridge University Botanic Garden, Boltenkov s.n. (cult.) | LT978558/ LT981300/ LT984450/ LT984482 |
| Sc2 (1) | H5 | United Kingdom, Hertfordshire, St. Albans, Boltenkov s.n. (cult.) | LT978540/ LT981282/ LT984432/ LT984465 |
| ZOR (1) | H4 | Armenia, Zorakert, Fayvush et al. 09-1696 (ERE) | LT627901/ LT628017/ LT628028/ LT628007 |
| Outgroup specimens | |||
| I. ser. Laevigatae | |||
| I. ensata | |||
| ZAR | Russia, Primorsky Krai, Zarubino, Boltenkov s.n. (VBGI) | LT627896/ LT628012/ LT628022/ LT628002 | |
| I. laevigata | |||
| ROS | Russia, Primorsky Krai, Roshchino, Pshennikova s.n. (cult.) | LT627897/ LT628013/ LT628024/ LT628003 | |
| I. pseudacorus | |||
| VLA | Russia, Vladivostok, Boltenkov s.n. (cult.) | LT627898/ LT628014/ LT628025/ LT628004 | |
| I. ser. Lacteae | |||
| I. lactea | |||
| ZAB | Russia, Zabaykalsky Krai, Kharanor, Chernova s.n. (IRK) | LT627854/LN871708/ LN871662/ LN871625 | |
| I. oxypetala | |||
| SHI | China, Shaanxi, Suyde, Kabanov s.n. (LE) | LT627844/ LT627950/ LT627975/ LT627911 | |
| I. tibetica | |||
| QHU | China, Qinghai, Riyue Xiang, Long et al. 60 (E) | LT627892/ LT627943/ LT627997/ LT627932 | |
| I. ser. Ruthenicae | |||
| I. uniflora | |||
| ANIS | Russia, Primorsky Krai, Anisimovka, Orlovskaya s.n. (VBGI) | LT627832/LN871684/ LN871640/ LN871604 | |
| ZKY | Russia, Kyrinsky District, Vologdina s.n. (cult.) | LT627902/ LT628018/ LT628029/ LT628008 | |
| I. subgen. Pardanthopsis | |||
| I. dichotoma | |||
| RDA | Russia, Amur Oblast, Baranova s.n. (cult.) | LT978555/ LT981297/ LT984447/ LT984483 | |
Notes:
N, number of analyzed individuals; H, haplotype; cult., cultivated. * Herbarium codes according to Thiers, 2020. Accession numbers in italics are reported in a previous study (Boltenkov et al., 2018).
DNA extraction, amplification, and direct sequencing of four non-coding cpDNA regions (trnS–trnG, trnL–trnF, rps4–trnSGGA, and psbA–trnH) follows Kozyrenko et al. (2004); Kozyrenko, Artyukova & Zhuravlev (2009). The cycle sequencing was accomplished on both strands and fragments were separated using a genetic analyzer ABI 3130 (Applied Biosystems, USA) in the Instrumental Centre of Biotechnology and Gene Engineering (Vladivostok, Russia). Sequences were deposited in the European Nucleotide Archive database; their accession numbers are available in Table 2.
Data analysis
The sequences of each cpDNA region obtained in this study and retrieved from the complete chloroplast sequence of I. sanguinea (KT626943) were aligned manually using the program SeaView v. 4 (Gouy, Guindon & Gascuel, 2010) and concatenated for each specimen. We included in the dataset indels and length variation in mononucleotide repeats because repeatability tests allowed us to exclude PCR errors. The haplotypes were identified based on combined DNA sequences using DnaSP v. 5 (Librado & Rozas, 2009). This program was also used to calculate the degree of divergence between cpDNA sequences based on nucleotide substitutions. A haplotype network was built using Network v. 4.6 (Bandelt, Forster & Röhl, 1999), treating each deletion/insertion, regardless of size as a single mutational event and using the median joining (MJ) algorithm with default settings. To reveal relationships between I. sanguinea, I. sibirica, and I. typhifolia, a haplotype network was also built using a dataset including psbA–trnH and trnL–trnF sequences obtained in our study and sequences of I. typhifolia retrieved from GenBank.
Phylogenetic analyses were performed on two datasets of combined sequences for four cpDNA regions studied (available at https://purl.org/phylo/treebase/phylows/study/TB2:S26635). The first one was composed of sequences from the I. subser. Sibiricae specimens obtained in the present study, haplotypes of seven taxa of I. sect. Limniris and I. dichotoma as outgroup. The second dataset was enlarged by the addition of psbA–trnH and/or trnL–trnF sequences for 13 accessions of the I. subser. Sibiricae species available in GenBank, and for these accessions, lacking portions of sequences (trnS–trn G and rps4–trnS regions) were coded as missing. Phylogenetic analyses were performed using Maximum Likelihood (ML) and Maximum Parsimony (MP) methods as implemented in PAUP v. 4.0b10 (Swofford, 2003). Bayesian Inference (BI) was conducted using MrBayes v.3.2.6 (Ronquist & Huelsenbeck, 2003) on the CIPRES portal (http://www.phylo.org/; Miller, Pfeiffer & Schwartz, 2010). For the MP analyses, gaps were coded according to (Simmons & Ochoterena, 2000), as implemented in the program FastGap v. 1.2 (Borchsenius, 2009). Optimal trees were found using a heuristic search with 1,000 random addition sequence replicates, starting trees obtained via stepwise addition, tree bisection and reconnection (TBR) branch swapping and the MulTrees option in effect. For ML and BI analyses, GTR + I + G model was selected according to the Akaike information criterion (AIC) using Modeltest v. 3.6 (Posada & Crandall, 1998). ML heuristic searches were done using the resulting model settings, 100 replicates of random sequence addition, TBR branch swapping and MULTrees option on. In BI, using the default prior settings, two parallel MCMC runs were carried out for ten million generations, sampling every 1,000 generations for a total of 10,000 samples. Convergence of the two chains was assessed, and the posterior probabilities (PP) were calculated from the trees sampled during the stationary phase. The robustness of nodes in ML and MP trees was tested using bootstrap with 1,000 replicates (bootstrap percentage, BP).
Results
Morphological data
Morphological comparison among the I. subser. Sibiricae species is provided in Table 3. The results showed overlap of I. sanguinea, I. sibirica, and I. typhifolia at the morphological level (Fig. 3, Table 3). The majority of characters were variable in this analysis (see Coefficient of variation in Table S2).
| Character (code) | I. sanguinea | I. sibirica | I. typhifolia | |
|---|---|---|---|---|
| Rosette leaf length, cm (LL) | 24–77 | 24–88 | 28–99 | |
| Rosette leaf width, cm (LW) | 0.2–0.7(1.1) | 0.2–0.8(1.1) | 0.2–0.4 | |
| Flowering stem height, cm (SH) | 23–82 | 22–99 | 35–74 | |
| Inflorescence structure (IS) | terminal head or occasionally with a lateral head | terminal head or with a lateral head | terminal head or occasionally with a lateral head | |
| Number of flowers (NF) | 1–3(4) | 1–4(6) | 1–3(4) | |
| Number of cauline leaves (NC) | (0)1–2(3) | (0)1–2(3) | 1–3 | |
| Cauline leaf length, cm (UL) | 4–13(25) | 3.5–13.5 | 4–9.5 | |
| Bract length, cm (BL) | 2–7 | 2.1–5.5 | 3–6 | |
| Pedicel length ,cm (PL) | 0.6–6.5 | 0.4–6 | 0.5–6 | |
| Flower colour (FC) | blue to violet with purple veins | blue to violet with purple veins | violet with purple veins | |
| Fruit length, cm (FL) | 1.7–7.7 | 1.5–4.2 | 2.3–5.5 | |
| Fruit shape (FS) | oblong-ellipsoidal | oblong-ellipsoidal or ellipsoidal | oblong-ellipsoidal | |
| Seed shape (SS) | semirounded or irregular, flat, thin, slightly glossy, brown | semirounded or irregular, flat, thin, slightly glossy, brown | nearly elliptical, flat, thin, slightly glossy, brown | |
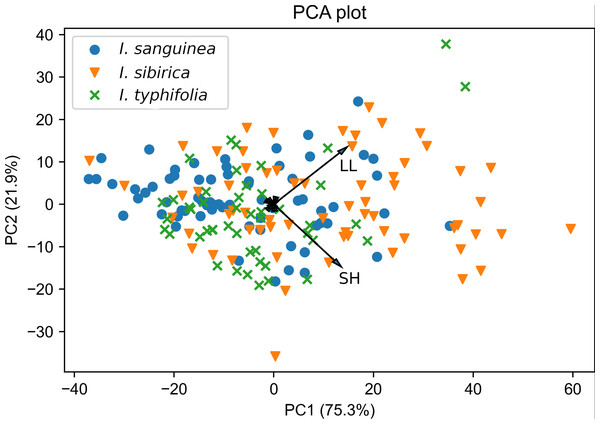
Figure 3: Principal components analysis of the Iris subser. Sibiricae species based on nine morphological characters.
Refer to Table 1 for character abbreviations.The result of PCA revealed three characters with high factor loadings (r ≥ 0.5) on the first three principal components. These are LL, SH and CL (see abbreviations in Table 1). Together, the first three components accounted for 99.2% of the total variation. The first two components explained 75.3% and 21.9% of the total variation, respectively.
The biplot of PCA for all those species illustrates the overlap between all specimens and significant morphological similarity (Fig. 3). Two characteristics SH and LL displayed the highest correlations with the first and second axis (corresponding values are r = 0.73 and r = 0.67), and the remaining one (CL) highly influenced the third axis (r = 0.93). Results of parametric and non-parametric ANOVA analysis to projected data on three principal components showed that mean (median in case of the non-parametric test) values do not differ significantly among the species. Corresponding statistics and p-values are: p-value = 0.21 and adjusted p-value = 0.63 for traditional ANOVA; p-value = 0.03 and adjusted p-value = 0.11 for Kruskal–Wallis test. However, being applied to the original plant characters, both parametric and non-parametric ANOVA tests showed significant differences of average values for I sanquinea, I. sibirica, and I. typhifolia. Our results showed that mean (in case of traditional ANOVA) and median (in case of Kruskal–Wallis test) values only for LL and possibly PL do not significantly differ among the considered species (Table S2). Thus, having likely different average values of morphometric characters, caused by environmental conditions and interspecific trait variability, these species can still be considered as indistinguishable in a generalized (PCA) factor space.
Molecular data
Among the 44 specimens studied, nine haplotypes (H1–H9) were identified based on nucleotide substitutions and indels detected across 3766 aligned positions of four cpDNA regions (Table 2). Four haplotypes (H6–H9) were unique, i.e., found in a single population: H6 in RLV population (Leningrad Oblast, Russia), H7 in population RMS from the Setun River valley (Moscow Oblast, Russia), H8 in population GBB from Georgia, while H9 was found in the plant Sc1 cultivated at the Botanic Garden of Cambridge University, the United Kingdom (UK). Five other haplotypes were detected in more than one accession, often from geographically distant locations in the I. subser. Sibiricae distribution range. The sequences of cpDNA regions obtained in our study were compared with those from the complete chloroplast sequence of I. sanguinea from the Republic of Korea (KT626943). Haplotype H1 found in accessions from two localities in Russia (RP1, RKY) and from three localities in Mongolia (BAD, MDB, and MKB), turned out to be identical with the haplotype of I. sanguinea from the Republic of Korea (KT626943). Specimens of populations RP3, RP4, and RP5 from Primorsky Krai, Russia shared haplotype H3, while populations ORL, RP2 (Primorsky Krai) and RCH (Amur Oblast, Russia) shared haplotype H2. Specimens from populations ALS, ALT, and ZOR (Armenia), GJP (Georgia), RKT (Karachay-Cherkess Republic, Russia), RRU (Udmurt Republic, Russia), and RKP (Kurgan Oblast, Russia) shared haplotype H4. Haplotype H5 was found in samples RKU (Kaluga Oblast, Russia), RPS (Pskov Oblast, Russia), and LAS (Austria) as well as in a cultivated plant Sc2 (UK). No specimen from the European part of the distribution range shared haplotypes with plants from the Asian part. The sequence divergence of cpDNA between plants from the European and Asian parts of the distribution range was very low (KS = 0.00056).
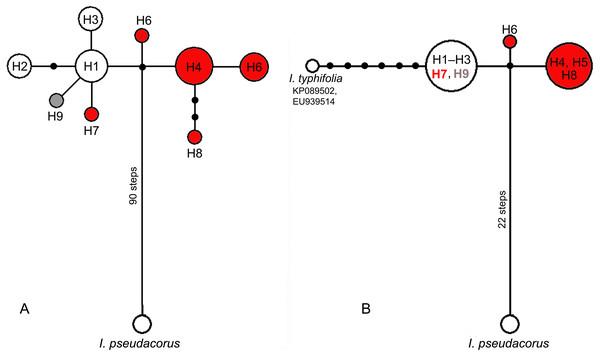
In the median network, all haplotypes formed one group (Fig. 4A) with a minimal divergence between each other (one to three mutational steps). Five haplotypes (H1–H3, H7, and H9) formed a star-like structure with haplotype H1 in the centre. This group composed of all haplotypes (H1–H3) from East Asian plants also included H7 from Eastern Europe and differed only by one substitution in the psbA–trnH region from all other haplotypes found in plants from the European range, namely haplotypes H4–H6 and H8. All haplotypes found across the I. subser. Sibiricae distribution range were closely related and derived from the same unsampled or extinct ancestral haplotype connected by many mutation steps with the haplotype of I. pseudacorus from I. ser. Laevigatae (Fig. 4A). A similar pattern was obtained in the network based on sequence data from the psbA–trnH and trnL–trnF regions, which included sequences of I. typhifolia retrieved from GenBank (Fig. 4B). In this network, all specimens from the Asian part of range share the common haplotype connected by six mutational steps with haplotype of I. typhifolia and by two steps with two haplotypes found in specimens from the European range.
Figure 4: Median-joining networks showing the relationships among cpDNA haplotypes of the Iris subser. Sibiricae species found in 27 localities across the distribution range including I. sanguinea sample from the Republic of Korea (KT626943) and I. pseudacorus as outgroup.
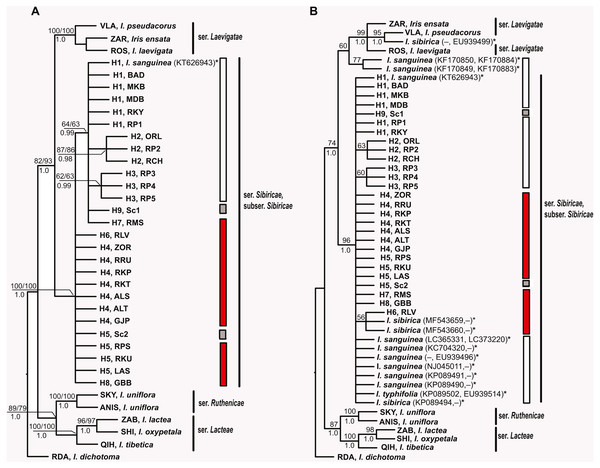
(A) The data are based on combined sequences of the trnS–trnG, trnL–trnF, rps4–trn SGGA, and psbA–trnH regions. (B) The data are based on combined sequences of the psbA–trnH and trnL–trnF regions including sequences of I. typhifolia retrieved from GenBank (KP089502, EU939514). Each circle represents a haplotype and the size of the circle is proportional to the number of population where that haplotype is found. Red circles –haplotypes found in plants from the I. sibirica distribution range; white circles –haplotypes found in plants from the I. sanguinea distribution range; grey circle –haplotype from cultivated plant S1. Black dots indicate intermediate haplotypes not observed in the sampling. Haplotype codes as in Table 2.MP, ML and BI analyses based on sequences of I. subser. Sibiricae obtained in the present study yielded similar topologies with few differences in node statistical supports (Fig. 5A). All Iris specimens clustered into highly supported (BP 100, 100%, PP 1.0) clades according to their affiliation to corresponding series of I. sect. Limniris. Haplotypes of all plants belonging to I. subser. Sibiricae formed a monophyletic highly supported clade (BP 100, 100%, PP 1.0) sister to the clade including species of I. ser. Laevigatae (BP 82, 93%, PP 1.0). Within the I. subser. Sibiricae clade, it was possible to distinguish a group including haplotypes H1–H3 from the Asian part of range, haplotype H7 from the Moscow Oblast (Russia), as well as haplotype H9 of the cultivated plant (Sc1), though this group received poor support in the MP and ML analyses (BP 63, 64%) and strong support only in BI analysis (PP 0.99). The overall topology of MP and BI trees (Fig. 5B) constructed with dataset including thirteen accessions of the I. subser. Sibiricae species retrieved from GenBank was largely similar to those of the trees described above (Fig. 5A). Ten of the thirteen additional accessions of I. sanguinea, I. sibirica, and I. typhifolia were placed together with all specimens of I. subser. Sibiricae in a monophyletic group (BP 100%, PP 1.0). However, the phylogenetic relationships within this clade were unresolved. Only one of three I. sibirica accessions (voucher Mosulishvili G99-12, RSA; see Wilson, 2009) and two (isolates ISD1 and ISD2, Lee & Park, 2013) of six accessions of I. sanguinea from the Republic of Korea were placed outside of the I. subser. Sibiricae clade but clustered with the I. ser. Laevigatae species (Fig. 5B). The sequence divergence (KS) calculated for two cpDNA regions between Korean accessions of I. sanguinea placed in the I. ser Laevigatae clade and I. sanguinea accessions placed in the I. subser Sibiricae clade was 0.009510 that was comparable with divergence between species in other series of I. sect Limniris (0.00451–0.01223; Boltenkov et al., 2018).
Figure 5: Phylogenetic analysis of Iris subser. Sibiricae.
(A) Strict consensus tree of the six equally most parsimonious trees resulting from MP analysis of combined plastid trn S–trnG, trnL–trnF, rps4–trnSGGA, and psbA–trnH sequences from 27 localities across the distribution range of Iris subser. Sibiricae including I. sanguinea sample from the Republic of Korea, KT626943 (Tree length of 429 steps, CI = 0.8228, RI = 0.8905). (B) Strict consensus tree of more than 600,000 equally most parsimonious trees resulting from MP analysis of the enlarged dataset including psbA–trnH and/or trnL–trnF sequences for 13 additional accessions of the I. subser. Sibiricae species retrieved from GenBank (Tree length of 469 steps, CI = 0.7655, RI = 0.8579). The numbers above and below branches indicate bootstrap values (> 50%) for MP/ML analyses and Bayesian posterior probabilities (>0.90) for BI analysis, respectively. Haplotype and locality codes correspond to those in Table 2. The asterisk (*) indicates species names and accession numbers of the sequences retrieved from GenBank. Bars indicate the geographical origin of the examined populations: white –East Asia; red –Europe and Western Siberia; grey –cultivated plants.Discussion
The overlapping of some previously considered diagnostic characters of I. sanguinea, I. sibirica, and I. typhifolia (see Fig. 3, Table 3) indicates that they constitute a group of morphologically very similar taxa, difficult to tell apart. We came to the conclusion that the key characters reported to distinguish I. typhifolia from I. sibirica are not stable and overlap among specimens attributed to either name.
Our examination of herbarium specimens and the analysis of the relevant literature revealed a wide range of variation in I. sanguinea and I. sibirica morphological characters. Key morphological characters discriminating I. sanguinea and I. sibirica are considered the features of the flowering stem structure. However, our data show that the flowering stems can be longer or shorter than the basal leaves, depending on the phenological phase, as well as simple or branched (Table 3). Skrypec & Odintsova (2017) also reported a high variability of the I. sibirica inflorescences structure. In our survey of herbarium specimens from the I. subser. Sibiricae distribution range, most plants had a flowering stem with terminal head of two flowers. In some parts of the I. subser. Sibiricae distribution range, plants with terminal and one lateral head are rarer (i.e., Omsk Oblast, Novosibirsk Oblast, and Buryatia Republic) or are the only ones (northern Kazakhstan, north of the European part of the Russia, Irkutsk Oblast, Zabaykalsky Krai, Sakha Republic, and Russian Far East). Previously, Poljakov (1958) indicated that the plants with terminal head is the typical of I. sibirica in northern Kazakhstan. Therefore, contrary to the general assumption of many botanists, inflorescence structure could not be a diagnostic key to distinguish species in I. subser. Sibiricae. In addition, our data showed that leaf width is variable in both I. sanguinea and I. sibirica, so it could not be used as a diagnostic character either. Differences of these characters observed may be the result of environmental conditions and the variability of characters within the species.
In the present study, we also failed to genetically distinguish between specimens collected in different localities of the I. subser. Sibiricae distribution range where I. sanguinea or I. sibirica are considered to occur (Figs. 4 and 5). Our analyses based on sequence variability in four non-coding regions of cpDNA showed an absence of clear differentiation between plants of I. sanguinea growing eastward Lake Baikal and I. sibirica distributed in Europe and Western Siberia. All specimens studied were closely related to each other and are clearly separated from other species in I. sect. Limniris. However the samples from the I. sanguinea distribution range together with a specimen RMS from European part of the range formed a distinct clade supported only in BI analysis (Fig. 5). Only one single point mutation in psb A–trn H distinguished these groups indicating their minimally differentiation. Nucleotide divergence of cpDNA between these groups (KS = 0.00056) is lower than between species in other series of I. sect. Limniris (0.00451–0.01223; Boltenkov et al., 2018) and comparable with divergence between populations of some Iris species, e.g., I. lactea (0.00037–0.00112; Boltenkov, Artyukova & Kozyrenko, 2016). The star-like structure of haplotype diversity also indicates an absence of deep phylogenetic split between plants from European and Asian parts of the I. subser. Sibiricae distribution range and is consistent with a rapid range expansion (Ferreri, Qu & Han, 2011).
In phylogenetic trees (Figs. 5A, 5B), all 44 specimens of Siberian irises studied as well as most accessions of I. subser. Sibiricae available in GenBank (including I. typhifolia) form a single monophyletic clade sister to the clade including species of I. ser. Laevigatae. Previously, the monophyly of the I. subser. Sibiricae species was also shown in phylogenetic study of Tillie, Chase & Hall (2000). In other studies, where the same one specimen (voucher Mosulishvili G99-12, RSA) was used as sole representative of I. sibirica, this specimen was embedded within the clade comprising species from I. ser. Laevigatae (Wilson, 2009; Mavrodiev et al., 2014) or I. ser. Lacteae (Jiang et al., 2018), thus making I. subser. Sibiricae polyphyletic. Crespo, Martínez-Azorín & Mavrodiev (2015) have pointed out that additional samples of I. sibirica should be sequenced to determine the phylogenetic position of this species at the infrageneric level. The specimen Mosulishvili G99-12 was confirmed as a misidentification (Carol Wilson & Marine Mosulishvili, 2020, pers. comm.). Only DNA material, but no herbarium voucher was collected by Mosulishvili from Kazbegi, north-eastern Georgia, in 1999. Moreover, it was noted (Mosulishvili, 2020, pers. comm.), that I. sibirica was never found near Kazbegi, while I. pseudacorus is common in this area. Other two samples of I. subser Sibiricae (isolates ISD1 and ISD2, Lee & Park, 2013) that had fallen into the clade of the I. ser Laevigatae species were of I. sanguinea from the Republic of Korea. Large divergence of these samples from all other samples of I. sanguinea from the Republic of Korea and other parts of the distribution range is comparable with divergence between different species of I. sect Limniris and the further studies are required to establish the species affiliation of these Korean samples. In this work, none of the studied specimens belonging to I. subser. Sibiricae fell within the I. ser. Laevigatae clade. Thus, our results clearly show that I. subser. Sibiricae is a monophyletic taxon that is strongly supported as sister to the I. ser. Laevigatae species.
The broad morphological variation, including inflorescence structure, observed in the group surveyed, together with the molecular results, point out to the difficulty in separating I. sanguinea at specific rank. Evidently, I. sibirica includes a set of morphotypes, but it remains homogeneous taxonomically, without possible recognition of infraspecific taxa or separate species, as evidenced by the molecular data obtained in this study. Therefore, we regard I. sanguinea, I. sibirica, and I. typhifolia as synonymous and formally propose a reduction of I. sanguinea and I. typhifolia to I. sibirica, which is the earliest legitimate name and has priority (Art. 11.3, Turland et al., 2018).
Taxonomic treatment
In the present study we confirm that I. subser. Sibiricae includes only a single variable species, I. sibirica. It is the most widespread Iris species, occurring from Central and Eastern Europe, including northeast Turkey, northern Kazakhstan, and Caucasus, to Siberia, East Asia (northern Mongolia, northern and eastern China, Korean Peninsula, and Japan), and the southern Russian Far East. It is found growing wild in moist meadows along river valleys. It is cultivated worldwide and sometimes naturalized. Morphologically, I. sibirica is distinct from I. subser. Chrysographes species by having shorter bracts (2–6 cm long), a much shorter perianth tube (no more than 0.5 cm long), and green basal leaves. The synonymic list of taxa specified in the present work, including types, is provided below.
Iris sibirica L., Sp. Pl. 1: 39. 1753. ≡ Iris pratensis Lam., Fl. Franç. 3: 498. 1779, nom. illeg. (Art. 52.1, Turland et al., 2018). ≡Biris sibirica (L.) Medik., Staatswirthschaftl. Vorles. Churpfälz. Phys.-Ökon. Ges. Heidelberg, 1: 257. 1791. ≡ Iris stricta Moench, Methodus, 2: 528. 1794, nom. illeg. (Art. 52.1). ≡ Iris angustifolia Salisb., Prodr. Stirp. Chap. Allerton: 44. 1796, nom. illeg. (Art. 52.1). ≡ Xiphion sibiricum (L.) Schrank, Flora 7(2, Beil.): 19. 1824. ≡ Xiphion pratense Parl., Nuov. Gen. Sp. Monocot.: 45. 1854. ≡ Limniris sibirica (L.) Fuss, Fl. Transsilv.: 637. 1866. ≡ Xyridion sibiricum (L.) Klatt, Bot. Zeitung (Berlin), 30: 500. 1872. – Limnirion sibiricum (L.) Opiz, Seznam: 5. 1852, nom. inval. (Art. 38.1). – Iris sibirica var. typica Maxim., Bull. Acad. Imp. Sci. Saint-Pétersbourg, 26: 519. 1880, nom. inval . (Art. 24.3). Type: [Specimen from a cultivated plant]. sibirica 9, HU [Horto Upsaliensis], Herb. Linnaeus (lectotype: designated by Altinordu & Crespo, 2016: 297, LINN! [LINN No. 61.20]).
= Iris orientalis Thunb., Trans. Linn. Soc. London, 2: 328. 1794, nom. illeg. (non Mill., Gard. Dict., ed. 8: Iris No. 9. 1768; Art. 53.1), syn. nov. ≡ Xiphion orientale Schrank, Flora 7(2, Beil.): 19. 1824. ≡ Iris sibirica var. orientalis (Schrank) Baker, J. Linn. Soc., Bot. 16: 139. 1877. ≡ I. extremorientalis Koidz., Bot. Mag. (Tokyo), 40: 330. 1926, nom. nov. (Art. 6.11). Type: Japan. [Note on the upper side]: Iris sibirica, Fl. jap. p. 33, Barin; [Note on the reverse side]: e Japonia, Thunberg s.n., Herb. Thunberg (lectotype: UPS [UPS-THUNB 1144, image!], designated here by E.V. Boltenkov).
= Iris sanguinea Hornem., Hort. Bot. Hafn. 1: 58. 1813, syn. nov. ≡ I. sibirica var. sanguinea (Hornem.) Ker Gawl., Bot. Mag. 39: t. 1604. 1814. ≡ Limniris sanguinea (Hornem.) Rodion., Bot. Zhurn. (Moscow & Leningrad), 92: 551. 2007. – Iris sanguinea Donn, Hort. Cantabrig., ed. 6: 17. 1811, nom. inval. (Art. 38.1) – I. sanguinea var. typica Makino, J. Jap. Bot. 6: 32. 1930, nom. inval. (Art. 24.3). Type: [Specimen from a cultivated plant]. [Handwriting 1]: Iris sanguinea, ex hort. bot. Hafn.; [Handwriting 2]: [Iris sanguinea ] Don., ad I. sibir [ica ]. L. ref. spr., Herb. Hornemann (lectotype: designated by Boltenkov, 2018: 178, C [C10022296, image!]).
= Iris sibirica var. haematophylla Besser, Flora, 17(1, Beibl.): 25. 1834, syn. nov. Type: [Specimen from a cultivated plant]. Iris (sibirica) haematophylla, Dahuria, Fischer s.n., Herb. Lindley (neotype: CGE! [CGE14724 ], designated here by E.V. Boltenkov).
= Iris typhifolia Kitag., Bot. Mag. (Tokyo), 48: 94. 1934, syn. nov. ≡ Limniris typhifolia (Kitag.) Rodion., Bot. Zhurn. (Moscow & Leningrad), 92: 551. 2007. Type: China. [Liaoning Province], Iris sibirica? …14 Aug. 3 [1928], K. Yamatsuta 60 (holotype: TI [image!]).
Conclusions
In Iris subser. Sibiricae, both morphological and geographical aspects are important to delimitate species. In this group, I. sanguinea, I. sibirica, and I. typhifolia have been recognized. However, analyses of morphological and molecular phylogenetic data may allow positioning the species among its relatives more exactly. In the case presented here, we reconstructed the phylogeny based on four non-coding regions of plastid DNA (trnS–trnG, trnL–trnF, rps4–trnSGGA, and psbA–trnH), and explored morphological characters to determine the relationship between species. At the same time, we once again showed that these regions are very informative for the taxonomy of irises, as they allow identifying species. Our results show that the morphological characters of I. sanguinea, I. sibirica, and I. typhifolia are overlaping. Phylogeny studies show that in accordance with the current circumscription, Iris subser Sibiricae is not polyphyletic. All the three species are nested together forming a well-supported monophyletic group (BP 100%, PP 1.0). It is thus concluded that I. sanguinea and I. typhifolia are conspecific with I. sibirica, a previously described species.
Supplemental Information
Sample information of the Iris subser. Sibiricae accessions from GenBank
A dash (–) indicates not available.
The results of the variance analysis of the Iris subser. Sibiricae species
M –mean (cm), S –standard deviation, C –coefficient of variation (%). The values in parentheses are adjusted p-values. Refer to Table 1 for character abbreviations.