COMPOSITIONS AND METHODS
FOR BAMBOO PULPING
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Patent Application Serial Nos. 61/433,153, filed January 14, 2011; 61/514,792, filed August 3, 2011; 61/514,797 , filed August 3, 2011; and 61/553,437, filed October 31, 2011, all of which are hereby incorporated by reference in their entireties for all purposes.
FIELD OF THE DISCLOSURE
[0002] Disclosed herein are compositions, systems and methods for bamboo pulping.
BACKGROUND OF THE DISCLOSURE
[0003] The subfamily Bambusoideae (of the family Poaceae), comprises both woody and herbaceous bamboos. At present roughly 120 genera of temperate and tropical woody bamboos are recognized. Bamboos are versatile plants with many different applications. It has been estimated that approximately 2.2 billion people worldwide use bamboo to some extent, and in 1985 the global revenue attributable to bamboo was estimated around U.S. $4.5 billion. The market for bamboo is also expanding. Bamboo shoots are a staple of Asian cuisine, and bamboo is found in a number of products including toothpicks, brooms, poles for viticulture and arboriculture, landscaping materials, parquet flooring, laminate materials, furniture, handicrafts and other household items. In addition, bamboo is becoming an important source of textile material as a component of paper production and as a source of structural timber.
[0004] Bamboo is considered an environmentally friendly "green" product. One of the characteristics that give bamboo its green reputation is its extremely rapid growth rate. Bamboo is the fastest growing woody plant in the world, achieving growth rates of well over three feet per day. It achieves this rate of growth in part because of its rhizome system, which is capable of providing a great deal of energy toward shoot growth.
[0005] With increasing burdens on land to produce food and biomass for energy and materials additional attention is place on faster growing materials for the pulp and paper industry. The Bamboo subfamily {Bambusoideae) are considered one of the fastest growing plants1. These evergreen, monocotyledonous grasses produce primary shoots which can grow as high as 40m and 30 cm in diameter in a growing season, where they mature over the
next several years, and nourish the plant rhizome system from which they grew from 2. Mature culms may be harvested for material between 3-5 years of maturity, when they have maximized their fiber content and structural rigidity. The growth rate of bamboo has been reported to be about 5-10 metric tons/hectare/year which is 2 times that of most trees2' 3.
[0006] Due to their high growth rate bamboo plants are already used extensively in Asia for timber and pulp and paper. In China, where there is a fiber shortage for the pulp and paper industry they currently produce about 1 million tons, primarily of mixed species. Although bamboo appears to be a promising biomass source, few plantations exist due to the high inherent cost of establishment. Bamboo plants flower infrequently primarily reproducing primarily through rhizomal propagation, which creates a shortage of seeds or creates high- cost root stock. Recent advances in micropropagation suggest the technical and economic barrier to be removed for large scale propagation, opening the way for single species bamboo plantations.
[0007] One interesting species for the pulp and paper is Moso Bamboo {Phyllostachys edulis which is also sometimes called Phyllostachys pubescens). This species which originates in China, where it still is a major species harvested for it culms to be used a material and its shoots which are edible2. This species requires a climate with annual precipitation of 120 to 180cm, a mean annual temperature of 13-20C, and a mean monthly temperature >0C which allow it to be grown in the Southeastern US4' 5. This giant species has culms with similar dimensions as pulpwood allowing the biomass to be handled in a similar manner for harvesting and chipping, which is essential to be utilized by the current pulp industry infrastructure4. The present application discloses compositions and methods for industrial applications of bamboo, and pulping, bleaching, and physical properties of bamboo plants compared to another fast growing woody species, hybrid poplar, Populus maximowiczii x nigra, another fast growing woody species currently used by the U.S. pulp industry.
[0008] Despite bamboo's rapid growth rate, it has other characteristics that make it a difficult crop to manage. Perhaps the greatest difficulty comes from the fact that many commercially important bamboos only flower at intervals of as long as 60-130 years. Compounding the difficulties of this long flowering cycle is the fact that many bamboos exhibit mass (or gregarious) flowering, with all plants in the population flowering simultaneously. For example, Phyllostachys bambusoides flowers at an interval of 130 years, and in this species all plants of the same stock flower at the same time, regardless of differences in geographic locations or climatic conditions. After flowering, the bamboo dies.
[0009] Bamboo's lengthy flowering interval and propensity for mass flowering makes it very difficult to obtain seeds for propagation. Compounding this problem is the fact that bamboo seeds, even when they are available, remain viable for no more than 3-6 months.
[0010] As a result of these difficulties with the propagation of bamboo by seed, bamboo typically is propagated by asexual techniques such as clump division and cutting. These asexual propagation techniques, however, are insufficient to meet projected world demand because both their capacity to produce mass scale production, and their practical efficiency, are too low. In addition many asexual propagation methods have the downside of failing to eliminate pathogens present in the parent plants.
[0011] A method to achieve large scale production of bamboo is highly desirable. Micropropagation (also known as tissue culturing with the terms used interchangeably herein), is an excellent method to achieve this aim.
[0012] Micropropagated plants are grown in vitro in sterile media. Typically, the media comprises a gelling agent, with the addition of various compounds such as nutrients, inorganic salts, growth regulators, sugars, vitamins and other compounds.
[0013] A benefit to tissue culturing plants is that the plants can be grown in a sterile environment so that they remain disease free. Other benefits include the ability to grow very large numbers of plants in a small space, the reduced water and nutrient needs of micropropagated plants, and the rapid multiplication of tissues that can in turn be used to yield more tissue culture material. Moreover micropropagation is very flexible and rapid upscaling is possible (within 1 year nearly one million plants can be produced from any genotype). Such short time frames and large numbers cannot be rivaled by any conventional method. Tissue culturing also provides for the production of high quality plants which are easy to transport and deliver.
[0014] Some papers have been published which address tissue culturing of bamboo. In practice, however {i.e., for large or mass scale propagation of bamboos), the methods described in these papers do not translate into commercially viable propagation systems.
[0015] The difficulties encountered in tissue culturing bamboo are high incidences of endogenous or surface contaminations and browning, factors related to dormancy or topophysis and hyperhydricity. The present disclosure provides media, systems and methods that overcome these difficulties allowing the commercial-scale asexual production of bamboo.
SUMMARY OF THE DISCLOSURE
[0016] The present invention provides compositions, methods and systems for the production of uniform bamboo plants that can be used for producing bamboo pulp and mixed bamboo/wood pulps.
[0017] The present invention provides uniform bamboo plants that can be used to produce large boles of similar dimensions to timber that can be handled using existing timber harvesting and chipping equipment.
[0018] The compositions, methods and systems of the present invention provide uniform bamboo material that pulps and bleaches similarly to hardwood, thereby enabling high throughput and low operating costs.
[0019] The rapid propagation of bamboo provided by the compositions, methods and systems of the present invention permit large-scale bamboo plantations that provide uniform feedstock at low establishment costs.
[0020] The uniform bamboo plants provided by the compositions, methods and systems of the present invention can be prepared with the same or similar kraft pulping and bleaching sequences typically used for hardwoods, thereby providing strong, high-brightness fibers suitable for many furnish blends.
[0021] In some embodiments, the disclosure provides bamboo plants, plant parts, plant tissues, plant cells, plant materials, plant compositions, and/or compounds derived from the bamboo plants, plant parts, plant tissues or plant cells. In some embodiments, the bamboo plants, plant parts, plant tissus, plant cells, and/or the materials, compositions, and compounds derived from the bamboo plants, plant parts, plant tissues and plant cells are used for the pulping and paper industries.
[0022] In some embodiments, the pulping and/or bleaching procedures are done at bench (i.e., small commercial or laboratory) scales or at industrial (i.e., large commercial or factory) scales.
[0023] In some embodiments, the disclosure provides bamboo biomass material that is exclusively or substantially uniform for pulping.
[0024] In some embodiments, the uniform bamboo biomass material used for pulping is derived from a pure stand. In some embodiments, the bamboo biomass material used for pulping is derived from a pure stand comprising a particular bamboo clone, a particular bamboo variety, a particular bamboo species or a particular bamboo genus.
[0025] In some embodiments, the particular bamboo genus exclusively or largely comprises germplasm of the genus of Phyllostachys, Fargesia, Pleioblastus, Sasa, Pleioblastus, Thamnocalamus, Chusquea, Bambusa, Dendrocalamus, Guadua, Gigantochloa, Melocanna, Ochlandra, or Schizostachyum.
[0026] In some embodiments, the particular bamboo species exclusively or largely comprises germplasm of the species of Phyllostachys Moso, Phyllostachys bissetti; Fargesia denudata; Pleioblastus fortunei; Sasa Veitchii; Pleioblastus viridistriatus; Thamnocalamus crassinodus; Chusquea Culeo "Cana Prieta "; Bambusa Old Hamii; Phyllostachys Atrovaginata; Dendrocalamus Asper; Guadua angustifolia; Arundinaria gigantea; Bambusa balcoa; Bambusa vulgaris; Bambusa vulgaris 'Vitatta '; Bambusa Oldhamii; Bambusa tulda; endrocalamus brandesii; Dendrocalamus asper; Dendrocalamus hamiltoni; Dendrocalamus giganteus; Dendrocalamus membranaceus; Dendrocalamus strictus; Gigantochloa aspera; Gigantochloa scortechini; Guadua culeata; Guadua aculeata 'Nicaragua '; Guadua amplexifolia; Guadua angustifolia; Guadua angustofolia bicolor; Guadua paniculata; Melocanna bambusoides; eohouzeaua dullooa (Teinostachyum) ; Ochlandra travancorica; Phyllostachys nigra; Phyllostachys nigra 'Henon '; or Schizostachyum lumampao.
[0027] In some embodiments, the particular bamboo variety exclusively or largely comprises germplasm of a specific bamboo variety. In some embodiments, the bamboo variety belongs to the species of Phyllostachys Moso, Phyllostachys bissetti; Fargesia denudata; Pleioblastus fortunei; Sasa Veitchii; Pleioblastus viridistriatus; Thamnocalamus crassinodus; Chusquea Culeo "Cana Prieta "; Bambusa Old Hamii; Phyllostachys Atrovaginata; Dendrocalamus Asper; or Guadua Angustifolia Arundinaria gigantea; Bambusa balcoa; Bambusa vulgaris; Bambusa vulgaris 'Vitatta '; Bambusa Oldhamii; Bambusa tulda; endrocalamus brandesii; Dendrocalamus asper; Dendrocalamus hamiltoni; Dendrocalamus giganteus; Dendrocalamus membranaceus; Dendrocalamus strictus; Gigantochloa aspera; Gigantochloa scortechini; Guadua culeata; Guadua aculeata 'Nicaragua '; Guadua amplexifolia; Guadua angustifolia; Guadua angustofolia bicolor; Guadua paniculata; Melocanna bambusoides; eohouzeaua dullooa (Teinostachyum); Ochlandra travancorica; Phyllostachys nigra; Phyllostachys nigra 'Henon '; or Schizostachyum lumampao.
[0028] In some embodiments, the uniform bamboo biomass material used for pulping is derived from a Phyllostachys Moso variety or a Phyllostachys edulis Moso ' variety.
[0029] In some embodiments, the bamboo plants used for pulping are plants grown in a natural environment, a plant grown in a cultivated area, and/or a plant grown in a growth facility (e.g., a greenhouse).
[0030] In some embodiments, the bamboo plants are propagated by natural pollination. For example, such bamboo plants are obtained from bamboo seeds.
[0031] In some embodiments, the bamboo plants are propagated by conventional macropropagation methods, such as vegetative propagation. Non-limiting examples of vegetative propagation include clump division (e.g., offsets planting and rhizome planting), whole culm cutting, layering, culm-segment cutting, branch cutting and macroproliferation. In some embodiments, the pure bamboo plant is propagated by micropropagation, such as tissue culturing.
[0032] In some embodiments, the bamboo plants of the pure bamboo stands of the present invention exclusively or largely comprise germplasm of one specific bamboo clone, variety, species, or genus. For example, the pure bamboo stand can exclusively or largely comprise bamboo plants of the germplasm of a specific Phyllostachys Moso clone or variety. In some embodiments, the bamboo plants of the pure bamboo stand comprise exclusively or largely of the germplasm of Phyllostachys Moso species. In some embodiments, the Phyllostachys Moso variety is a pure variety derived from micropropagation, for example, utilizing the micropropagation methods described herein.
[0033] The present disclosure further provides compositions, methods and sytems for propagating uniform bamboo plants for pulping. In some embodiments, the compositions, methods and systems are used for the micropropagation of uniform bamboo plants. In some embodiments, the compositions, methods and systems are used for micropropagation of a pure bamboo stand.
[0034] In some embodiments, the disclosure provides compositions, methods and systems for pulp production with an increased yield by using uniform bamboo plants and/or pure bamboo stands.
[0035] The present disclosure also overcomes the difficulties encountered in the commercial-scale asexual production of bamboo by providing effective media, kits, systems and methods for tissue culturing bamboo in order to produce uniform bamboo for pulping and other industrial uses.
[0036] In some embodiments the media for micropropagating uniform bamboo comprises meta-topolin or an analogue thereof and at least two other cytokinins wherein the media supports multiplication cycles for at least six months.
[0037] Another embodiment includes media for micropropagating uniform bamboo wherein said media comprises at least three cytokinins and supports multiplication cycles for at least six months.
[0038] Another embodiment includes media for micropropagating uniform bamboo wherein said media comprises at least one auxin and at least two cytokinins and supports multiplication cycles for at least six months.
[0039] Another embodiment includes media for micropropagating uniform bamboo wherein said media comprises at least two auxins and at least two cytokinins and supports multiplication cycles for at least six months.
[0040] Another embodiment includes media for micropropagating uniform bamboo wherein said media comprises at least two auxins and at least three cytokinins and supports multiplication cycles for at least six months.
[0041] Another embodiment includes media for micropropagating uniform bamboo wherein said media comprises meta-topolin or an analogue thereof and supports multiplication cycles for at least six months.
BRIEF DESCRIPTION OF THE DRAWINGS
[0042] Figure 1 depicts Moso bamboo chips produced in a disc chipper.
[0043] Figure 2 depicts Moso pulping liquor experiments impact on yield and kappa number.
[0044] Figure 3 depicts poplar pulping liquor experiments impact on yield and kappa number
[0045] Figure 4 depicts bleaching response of the laboratory cooks. Error bars represents a 95% confidence interval of 4 tests.
[0046] Figure 5 depicts physical properties of handsheets from the laboratory cooks. Error bars represents a 95% confidence interval of 4 tests.
[0047] Figure 6 depicts physical properties of handsheets made from Moso pulps.
[0048] Figure 7 depicts handsheet physical and optical properties.
DETAILED DESCRIPTION OF THE DISCLOSURE
Definition
[0049] As used herein, the verb "comprise" as is used in this description and in the claims and its conjugations are used in its non-limiting sense to mean that items following the word are included, but items not specifically mentioned are not excluded.
[0050] As used herein, the term "plant" refers to any living organism belonging to the kingdom Plantae (i.e., any genus/species in the Plant Kingdom). This includes familiar organisms such as but not limited to trees, herbs, bushes, grasses, vines, ferns, mosses and green algae. The term refers to both monocotyledonous plants, also called monocots, and dicotyledonous plants, also called dicots.
[0051] As used herein, the term "plant part" refers to any part of a plant including but not limited to the shoot, root, stem, seeds, stipules, leaves, petals, flowers, ovules, bracts, branches, petioles, internodes, bark, pubescence, tillers, rhizomes, fronds, blades, pollen, stamen, and the like. The two main parts of plants grown in some sort of media, such as soil, are often referred to as the "above-ground" part, also often referred to as the "shoots", and the "below-ground" part, also often referred to as the "roots".
[0052] As used herein, the term "a" or "an" refers to one or more of that entity; for example, "a gene" refers to one or more genes or at least one gene. As such, the terms "a" (or "an"), "one or more" and "at least one" are used interchangeably herein. In addition, reference to "an element" by the indefinite article "a" or "an" does not exclude the possibility that more than one of the elements are present, unless the context clearly requires that there is one and only one of the elements.
[0053] As used herein, the term "bamboo" refers to plants in the subfamily of Bambusoideae. Representative genus of bamboo is described in International Patent Application Publication No. WO2011100762, which is incorporated herein by reference in its entirety.
[0054] As used herein, the term "pulping" refers to the process of producing fibrous material from wood, plant, fiber crops, waste paper or any other suitable source by separating cellulose fibers. In some embodiments, the separating methods involve one or more chemical and/or mechanical processes.
[0055] As used herein, the term "pulp" refers to the plant material remaining after the pulping process.
[0056] As used herein, the phrase "pure stand" refers to a plant population consisting exclusively, substantially or largely of germplasm of one genus, one species, one variety, and/or one genotype. The plant population can be located in or obtained from any place or system suitable for growing plants, such as a growth chamber, a greenhouse, a shadehouse, a
field or anywhere else that plants can be grown and/or maintained. The plants can be grown in any suitable medium, including but not limited to soil, vermiculite, hydroponics, tissue culture media, etc.
[0057] As used herein, "monoculture" refers to the cultivation or growth of a single crop or organism especially on agricultural or forest land.
[0058] As used herein, the term "germplasm" refers to the genetic material with its specific molecular and chemical makeup that comprises the physical foundation of the hereditary qualities of an organism.
[0059] As used herein, the phrase "derived from" refers to the origin or source, and may include naturally occurring, recombinant, unpurified, or purified molecules. A nucleic acid or an amino acid derived from an origin or source may have all kinds of nucleotide changes or protein modification as defined elsewhere herein.
[0060] As used herein, the term "offspring" refers to any plant resulting as progeny from a vegetative or sexual reproduction from one or more parent plants or descendants thereof. For instance an offspring plant may be obtained by cloning or selfing of a parent plant or by crossing two parent plants and include selfmgs as well as the Fl or F2 or still further generations. An Fl is a first-generation offspring produced from parents at least one of which is used for the first time as donor of a trait, while offspring of second generation (F2) or subsequent generations (F3, F4, etc.) are specimens produced from selfmgs of Fl's, F2's etc. An Fl may thus be (and usually is) a hybrid resulting from a cross between two true breeding parents (true -breeding is homozygous for a trait), while an F2 may be (and usually is) an offspring resulting from self-pollination of said Fl hybrids.
[0061] As used herein, the term "cross", "crossing", "cross pollination" or "crossbreeding" refer to the process by which the pollen of one flower on one plant is applied (artificially or naturally) to the ovule (stigma) of a flower on another plant.
[0062] As used herein, the term "cultivar" refers to a variety, strain or race of plant that has been produced by horticultural or agronomic techniques and is not normally found in wild populations.
[0063] As used herein, the term "plant tissue" refers to any part of a plant. Examples of plant organs include, but are not limited to the leaf, stem, root, tuber, seed, branch, pubescence, nodule, leaf axil, flower, pollen, stamen, pistil, petal, peduncle, stalk, stigma, style, bract, fruit, trunk, carpel, sepal, anther, ovule, pedicel, needle, cone, rhizome, stolon, shoot, pericarp, endosperm, placenta, berry, stamen, and leaf sheath.
[0064] As used herein, the term "genotype" refers to the genetic makeup of an individual cell, cell culture, tissue, organism (e.g., a plant), or group of organisms.
[0065] As used herein, the term "hybrid" refers to any individual cell, tissue or plant resulting from a cross between parents that differ in one or more genes.
[0066] As used herein, the term "inbred" or "inbred line" refers to a relatively true- breeding strain.
[0067] As used herein, the term "population" means a genetically homogeneous or heterogeneous collection of plants sharing a common genetic derivation.
[0068] As used herein, the term "variety" or "cultivar" means a group of similar plants that by structural features and performance can be identified from other varieties within the same species. The term "variety" as used herein has identical meaning to the corresponding definition in the International Convention for the Protection of New Varieties of Plants (UPOV treaty), of Dec. 2, 1961, as Revised at Geneva on Nov. 10, 1972, on Oct. 23, 1978, and on Mar. 19, 1991. Thus, "variety" means a plant grouping within a single botanical taxon of the lowest known rank, which grouping, irrespective of whether the conditions for the grant of a breeder's right are fully met, can be i) defined by the expression of the characteristics resulting from a given genotype or combination of genotypes, ii) distinguished from any other plant grouping by the expression of at least one of the said characteristics and iii) considered as a unit with regard to its suitability for being propagated unchanged.
[0069] As used herein, kappa number is the amount of oxidizing agent (KMn04) consumed by 1 gram of pulp. It measures lignin content linearly and is approximately 6.7 times the lignin content (varies by species).
[0070] As used herein, kappa factor is the charge of chlorine or chlorine dioxide in the first bleaching stage given by Kappa Factor = Percent Equivalent Chlorine/Kappa No (with
Equivalent Chlorine = Chlorine dioxide wt/2.63). Therefore, pulps with higher lignin from the pulping get more chlorine dioxide in the first stage to remove it.
[0071] Kappa number can be measured according to NF ISO 302 standard.
[0072] Brightness can be measured according to NF ISO 3688 standard on the
Technidyne Colour Touch spectrometer.
[0073] Viscosity of the pulp can be measured according to T 230 om-04 standard. Pulping
[0074] The resources used to make pulp are referred to as pulpwood. Wood pulp comes from almost any type of hardwood and softwood. Non-limiting examples of softwood trees
include spruce, pine, fir, larch and hemlock, and non-limiting examples of hardwood trees include acacia, eucalyptus, aspen, maple, pacific albus, and birch.
[0075] A pulp mill is a manufacturing facility that converts wood chips or other plant fiber source into a thick fiber board which can be shipped to a paper mill for further processing. Pulp can be manufactured using mechanical, thermal, semi-chemical, fully chemical, or other hybrid manufacturing methods (kraft and sulfite processes). More detailed information of making pulp can be found in Rydholm, 1985, Pulping Process, John Wiley & Sons Inc., ISBN 9780471747932, incorporated herein by reference in its entirety. The finished product may be either bleached or non-bleached, depending on the customer and end-use requirements.
[0076] Wood and other plant materials used to make pulp contain three main components (apart from water): cellulose fibers (desired for papermaking), lignin (a three-dimensional polymer that binds the cellulose fibers together) and hemicelluloses, (shorter branched carbohydrate polymers). The aim of pulping is to break down the bulk structure of the fiber source, be it chips, stems or other plant parts, into the constituent fibers.
[0077] Chemical pulping achieves this by degrading the lignin and hemicellulose into small, water-soluble molecules which can be washed away from the cellulose fibers without depolymerizing the cellulose fibers (chemically depolymerizing the cellulose weakens the fibers). The various mechanical pulping methods, such as groundwood (GW) and refiner mechanical (RMP) pulping, physically tear the cellulose fibers one from another. Much of the lignin remains adhering to the fibers. Strength is impaired because the fibers may be cut. There are a number of related hybrid pulping methods that use a combination of chemical and thermal treatment to begin an abbreviated chemical pulping process, followed immediately by a mechanical treatment to separate the fibers. These hybrid methods include thermomechanical pulping, also known as TMP, and chemithermomechanical pulping, also known as CTMP. The chemical and thermal treatments reduce the amount of energy subsequently required by the mechanical treatment, and also reduce the amount of strength loss suffered by the fibers.
[0078] Mechanical pulp: Manufactured grindstones with embedded silicon carbide or aluminum oxide can be used to grind small wood logs called "bolts" to make stone groundwood pulp (SGW). If the wood is steamed prior to grinding it is known as pressure groundwood pulp (PGW). Most modern mills use chips rather than logs and ridged metal discs called refiner plates instead of grindstones. If the chips are just ground up with the plates, the pulp is called refiner mechanical pulp (RMP) and if the chips are steamed while
being refined the pulp is called thermomechanical pulp (TMP). Steam treatment significantly reduces the total energy needed to make the pulp and decreases the damage (cutting) to fibers. Mechanical pulps are used for products that require less strength, such as newsprint and paperboards.
[0079] Thermomechanical pulp: Thermomechanical pulp is pulp produced by processing wood chips using heat (thus thermo) and a mechanical refining movement (thus mechanical). It is a two stage process where the logs are first stripped of their bark and converted into small chips. These chips have a moisture content of around 25-30% and a mechanical force is applied to the wood chips in a crushing or grinding action which generates heat and water vapour and softens the lignin thus separating the individual fibers. The pulp is then screened and cleaned, any clumps of fiber are reprocessed. This process gives a high yield of fiber from the timber (around 95%) and as the lignin has not been removed, the fibers are hard and rigid.
[0080] Chemithermomechanical pulp: Wood chips can be pretreated with sodium carbonate, sodium hydroxide, sodium sulfite and other chemicals prior to refining with equipment similar to a mechanical mill. The conditions of the chemical treatment are much less vigorous (lower temperature, shorter time, less extreme pH) than in a chemical pulping process since the goal is to make the fibers easier to refine, not to remove lignin as in a fully chemical process. Pulps made using these hybrid processes are known as chemithermomechanical pulps (CTMP).
[0081] Chemical pulp is produced by combining wood chips and chemicals in large vessels known as digesters where heat and the chemicals break down the lignin, which binds the cellulose fibers together, without seriously degrading the cellulose fibers. Chemical pulp is used for materials that need to be stronger or combined with mechanical pulps to give a product different characteristic. The kraft process is the dominant chemical pulping method, with sulfite process being second. Historically soda pulping was the first successful chemical pulping method.
[0082] Recycled pulp is also called deinked pulp (DIP). DIP is recycled paper which has been processed by chemicals, thus removing printing inks and other unwanted elements and freed the paper fibers. The process is called deinking. DIP is used as raw material in papermaking. Many newsprint, toilet paper and facial tissue grades commonly contain 100% deinked pulp and in many other grades, such as lightweight coated for offset and printing and writing papers for office and home use, DIP makes up a substantial proportion of the furnish.
[0083] Organosolv pulping uses organic solvents at temperatures above 140 °C to break down lignin and hemicellulose into soluble fragments. The pulping liquor is easily recovered by distillation.
[0084] Steam exploded fiber is a pulping and extraction technique that has been applied to wood and other fibrous organic material. The resulting fibers can be combined with organic polymers to produce fiber composite materials. Alternatively, the fibers, along with other extracted substances, can be processed chemically or digested to produce ethanol and other useful substances.
[0085] The pulp produced can be bleached to produce a white paper product. The chemicals used to bleach pulp have been a source of environmental concern, and recently the pulp industry has been using alternatives to chlorine, such as chlorine dioxide, oxygen, ozone and hydrogen peroxide.
[0086] Market pulp is any variety of pulp that is produced in one location, dried and shipped to another location for further processing. Important quality parameters for pulp not directly related to the fibers are brightness, dirt levels, viscosity and ash content. Market pulp is sold on an air-dry basis which is by definition the weight of pulp at a 10% moisture content. True solids of the pulp are measured on a lot basis and used to correct the weight invoiced.
[0087] Dry lap pulp is the most common form to sell pulp. This is pulp formed to about 1000 gram/square meter sheet which is dried to about 10 % moisture content. It is normally delivered as sheeted bales of 250 kg. The reason to form and dry sheets is that it allows for long term storage, lowers transportation costs, and aids logistics. Wet-lap pulp can be formed in a similar process making a sheeted bale, without drying. This product is typically at approximately 50% moisture content and has a limited storage period..
[0088] Roll pulp or reel pulp is the most common delivery form of pulp to non traditional pulp markets. Fluff pulp is normally shipped on rolls (reels). This pulp is dried to 5 - 6 % moisture content. At the customer this is going to a comminution process to prepare for further processing.
[0089] Some pulps are flash dried. This is done by pressing the pulp to about 50%> moisture content and then let it fall trough silos that are 15-17 m high. Gas fired hot air is the normal heat source. The temperature is well above the char point of cellulose, but large amount of moisture in the fiber wall and lumen prevents the fibers from being incinerated. It is dried down to less than 10 % moisture and are compacted into bales using a piston press. This
process is done for pulps that have drainage properties that make sheet formation energy intensive.
[0090] In making the tissue papers, the bamboo is first pulped. Pulping processes such as the kraft (or sulfate) process and the sulfite process remove lignin and a portion of the hemicelluloses. The kraft process does less damage to cellulose fibers than the sulphite process, thereby producing stronger fibers, but the sulfite process makes pulp that is easier to bleach. The chemical pulping processes use a combination of high temperature and alkaline (kraft) or acidic (sulphite) chemicals to break the chemical bonds of the lignin.
[0091] In some embodiments, material fed into the digester is small enough to allow the pulping liquor to penetrate the pieces substantially completely. What is fed into a digester can be of a uniform size. Chips or cut plant material can go to a digester where they can be mixed in an aqueous solution of pulping chemicals, then heated with steam. In the kraft process the pulping chemicals can be sodium hydroxide and sodium sulfide. In the sulfite process the pulping chemical can be a mixture of metal (sodium, magnesium, potassium or calcium) or ammonium sulfite or bisulfite.
[0092] After a period of time in the digester (in one embodiment several hours), the chips or cut plant material breaks down into a thick porridge-like consistency that can be removed from the digester. In one embodiment, the material can be "blown" or squeezed from the outlet of the digester through an airlock. In this embodiment, the sudden change in pressure results in a rapid expansion of the fibers, separating them even more.
[0093] Extracted liquid can then be concentrated, burned and the sodium and sulfur compounds recycled in a recovery process. Clean pulp (stock) can then optionally be bleached. Dried pulp can be cut, stacked, bailed and/or shipped to another facility for whatever further process is needed. In one embodiment, the pulp is loaded onto rollers in the production of tissue paper.
[0094] Bleached kraft pulp and bleached sulfite pulp are used to make high quality, white printing paper. One of the most visible uses for unbleached kraft pulp is to make brown paper shopping bags and wrapping paper where strength is particularly important. A special grade of bleached sulfite pulp, known as dissolving pulp, can be produced and used to make cellulose derivatives such as carboxylmethylcellulose which are used in a wide range of everyday products from laxatives to baked foods to wallpaper paste.
[0095] In certain embodiments, plant material can be pretreated with sodium carbonate, sodium hydroxide, sodium sulfite and other chemicals prior to refining with equipment similar to a mechanical mill. The conditions of the chemical treatment are less vigorous
(lower temperature, shorter time, less extreme pH) than in a chemical pulping process, since the goal is to make the fibers easier to refine, not to remove lignin as in a fully chemical process. Pulps made using these hybrid processes are known as chemi-thermomechanical pulps (CTMP). Sometimes a CTMP mill is located on the same site as a kraft mill so that the effluent from the CTMP mill can be treated in the kraft recovery process to regenerate the inorganic pulping chemicals.
Pulping Bamboo
[0096] Prior to the present invention, the art of bamboo pulping was imprecise and the parameters for bamboo pulping had not yet been optimized for maximum pulp yield.
[0097] The present invention provides compositions, methods and systems for the pulping of bamboo that result in high yields of pulp.
[0098] In some embodiments and as explained in more detail elsewhere herein, the compositions, methods and systems for bamboo pulping provided by the instant invention include using pure stands of bamboo and/or bamboo monocultures to provide the bamboo for pulping. Prior to the present invention, pure stands and/or monocultures of bamboo were not available for pulping or other purposes and uses.
[0099] According to the present invention, pulping of bamboo includes cooking. In some embodiments, pulping bamboo further includes delignification; Still in some embodiments, pulping bamboo optionally includes brightening. The processes can be, without limitation, kraft processes or soda processes.
[00100] According to the present invention, pulping of bamboo includes kraft cooking; 02 delignification; chlorine dioxide exposure; hydrogen peroxide reinforced alkaline extraction; and optionally chlorine dioxide brightening. It is understood that one or more step of this process can be modified, substituted, or replaced by one skilled in the art. It is also understood that the methods disclosed herein are not limited to the sequence of the steps as described herein, and one skilled in the art can rearrange the sequence of each step as needed, if possible.
[00101] According to the present invention, production of bamboo pulp can be accomplished under a range of different cooking conditions, including as follows: cooking temperatures ranging from about 165°C to about 173°C; retention time at the cooking temperature ranging from about 65 minutes to about 80 minutes; H factor ranging from about 920 to 1626; active alkali (as NaOH) ranging from about 22.0% to about 30.0%; active alkali (as Na20) ranging from about 17.2% to about 23.4%; sulfidity ranging from about 32.0%> to
about 57.0%; effective alkali (as NaOH) ranging from about 16.3% to 25.2%; effective alkali (as Na20) ranging from about 12.8% to about 19.6%; and, the ratio of liquid to wood/bamboo (i.e., "LAV" or "L:W"; aka as the liquor to biomass ratio) ranging from about 4.0: 1.0 to about 5.0:1.0. While these ranges are representative of the actual values that can be used, generally a specific value for each variable is chosen to produce a particular batch of pulp, such as is demonstrated by the Examples provided herein.
[00102] For example, according to one embodiment of the present invention, pulping of bamboo includes kraft cooking at about 30%> to 40%> sulfidity, e.g., about 35% sulfidity, about 10% to 25% effective alkali, e.g., about 17% effective alkali, and about 800 to 1400 H- factor, e.g., about an 1100 H-factor; 02 delignification at about 70- 110 psig oxygen, e.g., about 90 psig oxygen; chlorine dioxide exposure at an about 0.12-0.25 kappa factor, e.g., about 0.18 kappa factor with a sulfuric acid pH adjust; hydrogen peroxide reinforced alkaline extraction at about 40°C - 60°C, e.g., about 50°C; and optionally chlorine dioxide brightening at about 0.4-0.8%, e.g., about 0.6% charge with an initial alkaline pH.
[00103] In another embodiment of the present invention, pulping of the bamboo includes kraft cooking at about 30%> to 40%> sulfidity, e.g., about 35% sulfidity, about 10% to 25% effective alkali, e.g., about 17% effective alkali, and an about 800 to 1400 H-factor, e.g., about 1100 H-factor with a max temperature of about 150°C-180°C, e.g., about 165°C (about 4: 1 Liquor to Wood ratio); 02 delignification at about 70- 110 psig oxygen, e.g., about 90 psig oxygen at about 80°C -120°C , e.g., about 100°C; chlorine dioxide exposure at an about 0.12-0.25 kappa factor, e.g., about 0.18 kappa factor with a sulfuric acid pH adjust for about 30min-60min, e.g., about 45min; hydrogen peroxide reinforced alkaline extraction at about 40°C - 60°C, e.g., about 50°C; and optionally chlorine dioxide brightening at about 0.4-0.8%), e.g., about 0.6% charge with an initial alkaline pH.
[00104] In another embodiment, pulping of the bamboo according to the present invention includes kraft cooking at about 30% to 40% sulfidity, e.g., about 35% sulfidity, about 10% to 25% effective alkali, e.g., about 17% effective alkali, and an about 800 to 1400 H-factor, e.g., about 1100 H-factor with a max temperature of about 150°C-180°C, e.g., about 165°C (about 4: 1 Liquor to Wood ratio); 02 delignification at 90 psig oxygen at about 80°C -120°C , e.g., about 100°C for 40min - 80 min, e.g., about 60 minutes; chlorine dioxide exposure at an about 0.12-0.25 kappa factor, e.g., about 0.18 kappa factor with a sulfuric acid pH adjust for about 30min-60min, e.g., about 45 minutes at about 40°C - 80°C, e.g., about 60°C; hydrogen peroxide reinforced alkaline extraction at about 40°C - 60°C, e.g., about 50°C; and optionally
chlorine dioxide brightening at about 0.4-0.8%, e.g., about 0.6% charge with an initial alkaline pH.
[00105] In another embodiment, pulping of the bamboo includes kraft cooking at about 30% to 40%) sulfidity, e.g., about 35% sulfidity, about 10%> to 25% effective alkali, e.g., about 17% effective alkali, and an bout 800 to 1400 H-factor, e.g., about 1100 H-factor with a max temperature of about 150°C-180°C, e.g., about 165°C (about 4/1 Liquor to Wood ratio); 02 delignification at about 70-110 psig oxygen, e.g., 90 psig oxygen at about 80°C -120°C, e.g. , about 100°C for 40min-80min, e.g., about 60 minutes; chlorine dioxide exposure at an about 0.12-0.25 kappa factor, e.g., about 0.18 kappa factor with a sulfuric acid pH adjust for about 30min-60min, e.g., about 45min at about 40°C - 80°C, e.g., about 60°C (10% esc); hydrogen peroxide reinforced alkaline extraction at about 40°C - 60°C, e.g., about 50°C; and optionally chlorine dioxide brightening at about 0.4-0.8%, e.g., about 0.6% charge with an initial alkaline pH.
[00106] In one embodiment, the Kraft cooking conditions include using an effective alkali ranges from about 16.0% to about 18.0%; sulfidity from about 25% to about 30%>; an H- Factor from about 1000 to about 1200; a maximum cooking temperature (i.e., T max) of about 165°C; and a liquor to biomass ratio of about 4.0: 1 to 5.0: 1. The resulting bamboo pulp from using such cooking parameters has a Kappa number ranging from about 20 to about 25; and, a screened yield ranging from about 42% to about 44%. The typical "hardwood bleaching" from such bamboo pulp has a brightness ranging from about 85 to about 88; and, a CED viscosity greater than about 20 cP. The fiber morphology of such bamboo pulp has a coarseness ranging from about 11.0 mg/lOOm to about 12.0 mg/lOOm; a LWA fiber length ranging from about 1.1 mm to about 1.3 mm; and, a LWA fines ranging from about 20.0% to about 30.0%.
[00107] In another embodiment, pulping of bamboo includes using an effective alkali of about 17%; sulfidity of about 35%, an H-Factor of about 1100; a maximum temperature of about 165°C; and a liquor to biomass ratio of about 5: 1.
[00108] Following the procedures of the present invention, bamboo pulp yields (measured as screened or unscreened yield) from about 32.1% to about 50% or more can be expected after cooking and prior to bleaching.
[00109] When bleaching of bamboo pulp is utilized, according to the present invention a brightness of at least >80 can be achieved. In some embodiments of the present invention, a brightness of >85 can be achieved. In some embodiments of the present invention, a
brightness of >90 can be achieved. In still other embodiments of the present invention, a brightness of >95 can be achieved.
[00110] The methods and bamboo pulps of the present invention can be used for various manufacturing processes and products, including but not limited to paper and tissue paper manufacturing methods such as those disclosed in, for example, US Patent or Patent Publication Nos. 2010/020560; 2010/0163664; 2009/0314444; 2003/0000665; and 2009/0312536, each of which is incorporated by reference in its entirety.
[00111] The methods and bamboo pulps of the present invention can also be beneficially used with other various technologies, products and methods, such as those disclosed in the following US Patent Publication Nos.: US Patent Publication Nos. 20100043989, 20090056892, 20080097875, 20070102127, 20060243405, 20060201841, 20060191657, 20060086472, 20060027590, 20050241789, 20050136097, 20050123661, 20050067125, 20040118537, 20040118536, 20040118535, 20040118532, 20040099392, 20040089429, 20040018369, 20030203231, 20030155089, 20030135181, 20030131961, 20030126028, 20030121633, 20030121628, 20030118847, 20030056917, 20020112832, 20020088578, each of which is incorporated by reference in its entirety. Following the pulping processes of the present invention, pulped bamboo material can be loaded onto rollers for paper making. These techniques are well known to those skilled in the art.
[00112] Following the pulping processes of the present invention, pulped bamboo material can be loaded onto rollers for paper making. These techniques are well known to those of ordinary skill in the art.
Uses of Bamboo
[00113] As stated previously, there are many uses for bamboo compositions produced according to the methods and compositions provided according to the present invention. In addition to or complementing those uses described elsewhere herein, non-limiting examples of uses and products made from bamboo produced according to the media, systems and methods disclosed herein include:
[00114] Exemplary Paper Types: Freesheet; Stock; Acid- free; A4; Board; Bond; Book; Bristol; Carbonless; Catalog; Coated; Cover; Dual-Purpose Bond; Duplex; English Finish; Equivalent; Fine; Free Sheet; Grain Long; Grain Short; Groundwood; Kraft; Lightweight; News Print; Publishing; Rag; Recycled; Tag; Uncoated; Virgin; Absorbent; Acid; Album; Albumin; Alkaline; Bank Note; Tissue; Toilet; Towels; Fluff; Card Stock; one-time carbon (OTC); optical character recognition (OCR); Tissue Overlay; and Napkins.
[00115] Exemplary Pulp Types: Air-dried; Alpha; Bamboo; Bisulfate; Sulphite; Bleached; Chemical Cellulose (Dissolving ); Fluff; Fodder; Free; Fully Bleached; Hard; High Alpha Cellulose; Groundwood; Hot Groundwood; Jute; Knotter; Kraft; Long Fiber; Baled; Rolled; Market; Non- Wood; Board; Pressurized Groundwood;; Rag; Recycled; Reinforcement; Secondary; Semi-alkaline; Semi-bleached; Semi-chemical; Short Fiber; Soda; Specialty; Sulfate; Thermochemical; Unbleached; Viscose; and Wood.
[00116] Board/Containers: Linerboard; Containerboard; Cardboard; old corrugated containers (OCC); and Paperboard.
[00117] Wood: Structural Wood Panels (including Structural Plywood; Oriented Strand Board; Structural Composite Panels)'; Glued Laminated Timber; Structural Composite Lumber (including Laminated Veneer Lumber; Parallel Strand Lumber; Oriented Strand Lumber); Prefabricated Wood I-Joists; Floor Joints; Railroad Ties; Flooring; and Composites (including Auto; Aero; Musical).
[00118] Textiles: Feedstock; Filament Yarn; Knitted Fabric; Knitting; Narrow Width Fabric; Non Woven Fabric; Spun Yarn; Woven Fabric; Viscose Rayon; Batting; Ginned Fiber; and Cloth.
[00119] Textile Products: Clothing; Towels; Sheets/Bedding; Pillows; Curtains
[00120] Food sources: Shoots; and any direct or bi-product for food consumption by animals and humans.
[00121] Consumer Goods: Animal Feed; Carpeting; Light Bulbs; Household Cleaning Products; Chopsticks & Toothpicks; Cleaning Brooms; Bicycles; Wheel Chairs; Fishing rods; Beer; Liquor; Pharmaceuticals; Cosmetics; Soap/Shampoo; Kitchenware; Crafts; Furniture; Nutraceuticals; Paper cups; Paper plates; and Diapers.
[00122] Energy & Bioenergy: Charcoal; Insulation; Feedstock; and Biomass.
Using a Pure Stand and/or Monoculture of Bamboo for Pulping
[00123] Without wishing to be bound by any particular theory, many unexpected advantages can be achieved by using a pure stands and/or monocultures of bamboo as a pulping source according to the present invention, particularly when compared to using pulping methods conducted not using a pure stand and/or monoculture.
[00124] Biomass as a raw material contains significant chemical, physical and anatomical variability. During the processing of said biomass material into commodity products such as pulp it is desired to minimize the variability as much as possible. Doing so both improves the
quality of the raw material through reduction of variability and reduces cost through process optimization.
[00125] Bamboo pulp is currently made from wild stands composed of mixed bamboo genera, species, strains and/or varieties. The resulting mixed bamboo material has high variability and results in increased production costs as the cooking and bleaching cannot be optimized for such raw, non-uniform and variable material.
[00126] Use of wood products in industrial processes and commercial uses have been improved through use of plantations of a single species (eucalyptus, pine, etc.), strains and/or variety. Unfortunately, prior to the present invention, this was not practical for bamboo production as bamboo was extremely difficult to propagate through seeds (flowers very infrequently) or rhizome cuttings. Thus, use of a single bamboo species, strain and/or variety for large-scale commercial purposes was difficult and cost-prohibitive prior to the present invention.
[00127] This invention couples the use of micropropagation that allows for single strain (e.g., a pure stand) propagation in an affordable manner with the concept of creating monoculture plantations to be used for the commercial purposes, such as for the pulp and paper industry.
[00128] Additionally, according to the present invention, the use of single species, strain and/or variety of bamboo plantations allows for plants to be selected with the best processing and performance properties and then quickly propagated to a commercial scale.
[00129] Since, if desired, bamboo could also be cooked and bleached similar to hardwood species, such as poplar, one could co-cook bamboo with wood chips as a means to incorporate bamboo into existing processes and uses without having to do separate processing. In this scenario, bamboo chips could be blended with hardwood chips prior to loading the material in the digester. The resulting pulp could be washed and bleached and used as a blend for making paper. This would be especially beneficial for the initial inclusion of small percentages of bamboo into pulp and paper production.
[00130] In some embodiments, the pure bamboo stand comprises at least about 95%, at least about 95.5%, at least about 96%>, at least about 96.5%>, at least about 97%, at least about 97.5%, at least about 98%, at least about 98.5%, at least about 99%, at least about 99.5%, at least about 99.8%>, at least about 99.9% or 100% germplasm of one specific bamboo species, strain or variety, for example, the germplasm of a specific Phyllostachys Moso variety.
[00131] In some embodiments, the Phyllostachys Moso variety is a pure variety derived from micropropagation, for example, the micropropagation methods described herein.
[00132] In some embodiments, the pure bamboo stand comprises no more than about 5%, no more than about 4.5%, no more than about 4%, no more than about 3.5%, no more than about 3%, no more than about 2.5%, no more than about 2%, no more than about 1.5%, no more than about 1%, or no more than about 0.5%, no more than about 0.2%, no more than about 0.1%, or 0.0% of germplasm of one or more other bamboo species, strains an/or varieties, for example, the germplasm of one or more other Phyllostachys Moso varieties.
[00133] In some embodiments, the bamboo population used for pulping is a pure bamboo population comprising at least about 95%, at least about 95.5%, at least about 96%>, at least about 96.5%), at least about 97%, at least about 97.5%, at least about 98%, at least about 98.5%, at least about 99%, at least about 99.5%, at least about 99.8%, at least about 99.9% or 100%) of one specific bamboo variety, for example, the Phyllostachys Moso variety.
[00134] In some embodiments, the Phyllostachys Moso variety used for pulping is a pure variety derived from micropropagation, for example, the micropropagation methods described herein.
[00135] In some embodiments, the pure bamboo population used for pulping comprises no more than about 5%, no more than about 4.5%, no more than about 4%, no more than about 3.5%, no more than about 3%, no more than about 2.5%, no more than about 2%, no more than about 1.5%, no more than about 1%, no more than about 0.5%, no more than about 0.2%, no more than 0.1% or 0.0% of one or more other bamboo variety, for example, one or more other Phyllostachys Moso variety.
[00136] In some embodiments, the bamboo population used for pulping is a pure bamboo population comprises at least about 95%, at least about 95.5%, at least about 96%, at least about 96.5%), at least about 97%, at least about 97.5%, at least about 98%, at least about 98.5%, at least about 99%, at least about 99.5%, at least about 99.8%, at least about 99.9% or 100%) of one specific bamboo species, for example, the Phyllostachys Moso.
[00137] In some embodiments, the Phyllostachys Moso is a pure species derived from micropropagation, for example, the micropropagation methods described herein.
[00138] In some embodiments, the pure bamboo population comprises no more than about 5%, no more than about 4.5%, no more than about 4%, no more than about 3.5%, no more than about 3%, no more than about 2.5%, no more than about 2%, no more than about 1.5%, no more than about 1%, no more than about 0.5%, no more than about 0.2%, no more than about 0.1% or 0.0% of one or more other bamboo species, for example, one or more other non-Phyllostachys Moso species.
[00139] In some embodiments, the bamboo population used for pulping is a pure bamboo population comprises at least about 95%, at least about 95.5%, at least about 96%>, at least about 96.5%), at least about 97%, at least about 97.5%, at least about 98%>, at least about 98.5%), at least about 99%, at least about 99.5% of one specific bamboo genus, at least about 99.8%), at least about 99.9% or 100%, for example, of the Phyllostachys genus.
[00140] In some embodiments, the Phyllostachys genus is a pure genus derived from micropropagation, for example, the micropropagation methods described herein.
[00141] In some embodiments, the pure bamboo population comprises no more than about 5%, no more than about 4.5%, no more than about 4%, no more than about 3.5%, no more than about 3%, no more than about 2.5%, no more than about 2%, no more than about 1.5%, no more than about 1%, no more than about 0.5% of one or more other bamboo genus, no more than 0.2%, no more than 0.1% or 0.0%, for example, one or more other non- Phyllostachys genus.
[00142] In some embodiments, the critical silica data for Moso bamboo ranges from about 1.0% to 2.0%; while that for Henon bamboo ranges from about 2.0% to 3.0%. In certain embodiments, the critical silica data for Moso bamboo is about 1.3% and that for Henon bamboo is about 2.4%.
Bamboo Propagation
[00143] Bamboos are versatile plants with many different applications. They are a staple of Asian cuisine and are found in a number of products including toothpicks, brooms, poles for viticulture and arboriculture, landscaping materials, parquet flooring, laminate materials, furniture, handicrafts and other household items. In addition, bamboo is becoming an important source of textile material as a component of paper production and as a source of structural timber.
[00144] Bamboo is considered an environmentally friendly "green" product. One of the characteristics that gives bamboo its green reputation is its extremely rapid growth rate. Despite bamboo's rapid growth rate, however, it has other characteristics that make it a difficult crop to manage including its long flowering cycle and tendency to exhibit mass (or gregarious) flowering.
[00145] Bamboo can be propagated by many methods, such as the conventional vegetative propagation, seed propagation, or micropropagation. Non-limiting examples of conventional vegetative propagation include clump division (e.g., offsets planting and rhizome planting; whole culm cutting, layering, culm-segment cutting, branch cutting and macroproliferation.
[00146] Clump divisions: this is the traditional, and perhaps the most generally prevalent method of vegetative propagation (McClure 1966). Clump divisions are generally done in two ways - offset planting, and rhizome planting.
[00147] Culm cutting: Generally, culm segments of bamboos of 1, or usually 2-3 nodes bearing healthy buds or branches, have been used for propagation. The branches on each culm segments are generally pruned to a length of less than 25 cm and no foliage is retained. Such cuttings are usually set upright or at an angle, with at least one node well covered. Different bamboo varieties and plants respond with varying success to propagation by culm cuttings. One can cut off and bury complete culm sections of the desired bamboo. On can select a mature culm 2 or 3 years old, cut it into sections 4 to 6 feet long, and cut back all the branches leaving a single bud on each branch. One can leave the largest branch at each node(one per node). These large branches should be selected so that they are all in the same orientation on the culm, so they will all stick upwards when the culm is buried. One can then dig a trench long enough for the culm about 4 to 6 inches deep, bury the culm section with the longer branches sticking up above the soil, flood with water daily for a week, then twice a week for a couple more weeks. After that water often enough to keep the soil damp. After about six months one should be able to carefully dig up the entire buried culm with roots and rhizomes intact, cut the culm on either side of each rooted node and pot or bag the rooted sections and grow in a protected environment until large and strong enough to be replanted in its permanent home.
[00148] Layering: the layered stem when rooted is detached to become a new plant. Three layering procedures for bamboos have been described (McClure 1966). (1) Ground or Simple layering: Either a whole culm or only the branch bearing part of it is bent down to the ground and into a shallow trench, fastened in place by means of hooked or crossed stakes, and covering it with suitable propagating medium. (2) Stump layering: The 1-2 node stumps of severed culms are covered with a suitable propagating medium. (3) Air-layering or marcotting: A culm is kept erect, and the base of each branch complement in the mid-culm range is surrounded with a suitable propagating medium, held in place by a suitable receptacle.
[00149] Macroproliferation of bamboo seedlings: Several methods of vegetative propagation are common in many grasses, e.g. use of tillers, culms, rhizomes or stolons (Langer and Ryle 1958). Like many other grasses, bamboo has the inherent proliferating capacity to reproduce itself probably due to its long interseeding period. By utilizing this habit, an interesting
technique has been developed by Banik (1987a) for multiplication of a seedling through the rhizome separation method.
[00150] Propagation through seed: bamboo plants can also be propagated through seeds. The seeds can be produced naturally in natural environment, or produced by controlled pollination.
Bamboo Micropropagation
[00151] Embodiments disclosed herein provide for the micropropagation or tissue culturing (these terms are used interchangeably herein) of bamboo on a commercial scale.
[00152] Particular embodiments disclosed herein utilize media comprising at least three cytokinins. Additional embodiments utilize media comprising the cytokinin meta-topolin or an analogue thereof in combination with at least two other cytokinins. Additional embodiments utilize media comprising at least one auxin and at least two cytokinins. Additional embodiments utilize media comprising at least two auxins and at least two cytokinins. Additional embodiments utilize media comprising meta-topolin. Additional embodiments utilize media comprising at least two auxins and at least three cytokinins. The embodiments disclosed herein can be used to micropropagate all species of bamboo generally and in particular can be used to micropropagate species belonging to the genus Arundinaria, Bambusa, Borinda, Chusquea, Dendrocalamus, Fargesia, Guadua, Phyllostachys, Pleioblastus and Thamnocalamus.
[00153] Micropropagated plants are grown in vitro in sterile media. The sterile media can be liquid, semi-solid, or solid, and the physical state of the media can be varied by the incorporation or removal of one or more gelling agents. Any gelling agent known in the art that is suitable for use in plant tissue culture media can be used. Agar is most commonly used for this purpose. Examples of such agars include Agar Type A, E or M and Bacto® Agar (Becton Dickinson & Co.). Other exemplary gelling agents include carrageenan, gellan gum (commercially available as PhytaGel™ (Sigma-Aldrich), Gelrite® (Sigma-Aldrich) and Gelzan™ (Caisson Labs)), alginic acid and its salts, and agarose. Blends of these agents, such as two or more of agar, carrageenan, gellan gum, agarose and alginic acid or a salt thereof also can be used.
[00154] Typically, the media comprises a gelling agent, with the addition of various compounds such as nutrients, inorganic salts, growth regulators, sugars, vitamins and other
compounds. Other media additives can include, but are not limited to, amino acids, macroelements, iron, microelements, inositoland undefined media components such as casein hydrolysates, or yeast extracts. For example, the media can include any combination of NH4NO3; KN03; Ca(N03)2; K2S04; MgS04; MnS04; ZnS04; CuS04; CaCl2; Kl; CoCl2; H3B03; Na2Mo04; KH2P04; FeS04; Na2EDTA; Na2H2P04; myo-inositol; thiamine; pyridoxine; nicotinic acid; glycine; riboflavin; ascorbic acid; silicon standard solution; β- naphthoxyacetic acid (NAA); indole butyric acid (IBA); 3-indoleacetic acid (IAA); benzylaminopurine (BAP); 6-Y-Y-(dimethylallylamino)-purine (2ip); sugar; agar; carrageenan, graphite and charcoal.
[00155] Examples of plant growth regulators include abscisic acid (ABA), triacontanol, phloroglucinol, auxins and compounds with auxin-like activity, cytokinins and compounds with cytokinin-like activity. Exemplary auxins include 4-fluorophenoxyacetic acid (FA), 2,4,5-trichlorophenoxyacetic acid (2,4, 5-T), 3-bromooxindole-3-aceitc acid, 4- bromophenoxyacetic acid, dicamba, p-chlorophenoxyacetic acid (CPA) indole-3-propinoic acid (IP A), 2,4-dichlorophenoxyacetic acid (2,4-D), indole-3 -butyric acid (IBA), indole-3- acetic acid (IAA), picloram and combinations thereof. Exemplary cytokinins include meta- topolin, thidiazuron, N-(2-chloro-4-pyridyl)-N-phenylurea (CCPU), 1,3-diphenylurea (DPU), adenine hemisulfate, benzyladenine, dimethylallyladenine, kinetin, zeatin, riboside, adenosine, meta-topolin riboside, meta-topolin-9-glucoside, ortho-topolin, ortho-topolin riboside, ortho-topolin-9-glucoside, para-topolin, para-topolin riboside, para-topolin-9- glucoside, ortho-methoxytopolin, ortho-methoxytopolin riboside, meta-methoxytopolin, meta-methoxytopolin riboside, meta-methoxytopolin-9-glucoside and combinations thereof as well as plant extracts having cytokinin-like activity, such as coconut water, banana powder, malt extract, pinapple powder or tomato powder.
[00156] Gibberellic acid also can be included in the media. A sugar or combination of sugars can be included in the media and can serve as a carbon source. Such sugars are known to those of ordinary skill in the art. Exemplary sugars include fructose, sucrose, glucose, maltose, galactose mannitol and sorbitol or combinations thereof. Other exemplary additives (with suggested but non-limiting functions) include polyamines (regeneration enhancer); citric acid, polyvinylpyrodine (PVP) and sodium thiosulfate (anti-browning agents); CaN03 or calcium gluconate (hyperhydricity reducer); paclobutrazol or ancymidol (multiplication enhancer); acetyl salicylic acid (ethylene inhibitor) and p-chlorophenoxyisobutyric acid (PCIB) and triiodobenzoic acid (TIB A) (anti-auxins).
[00157] In particular embodiments, basal media can be Murashige and Skoog (MS). Suitable nutrient salts also include, without limitation, Anderson's Rhododendron, Chu's N-6, DKW, Gamborg's B-5, Hoaglands No. 2, Kao & Michayluk, Nitsch & Nitsch, Schenk and Hildebrant, Vacin and Went, Whites and WPM, available from commercial sources such as Caisson Laboratories, Inc or Phytotechnology Laboratories..
[00158] Disclosed herein are specialized media, kits, systems and methods that allow the successful tissue culturing of bamboo on a commercial scale. Certain media described herein include a combination of at least two cytokinins or meta-topolin and/or an analogue thereof in combination with at least one other cytokinin. Other media include at least one auxin and at least two cytokinins. Additional media include at least two auxins and at least two cytokinins. Additional media include at least two auxins and at least three cytokinins. While certain embodiments utilize meta-topolin, thidiazuron, NAA, IBA, BAP, 2ip, CCPU or DPU, other related compounds, referred to as analogues herein, can also be successful.
[00159] Compounds useful according to the present disclosure include meta-topolin analogues having a general formula
wherein W is an aryl or heteroaryl;
Pv1 is substituted or unsubstituted alkyl wherein any C in the alkyl can be substituted with O,
N or S;
each R2 is independently H, OH, Ci-C6 alkyl, Ci-C6 alkylene, Ci-C6 alkynyl, halogen, cyano,
Ci-C6 alkyloxy, aryl or heteroaryl each optionally substituted with a Ci-C6 alkyl, SH, NHR3, C02R3 or halogen;
R3 is H, OH, Ci-C6 alkyl, Ci-C6 alkylene, Ci-C6 alkynyl, halogen, carboxylic group, ester group, aldehyde or cyano;
r is O to 8.
wherein a dashed line represents the presence or absence of a bond;
XI -X is each independently selected from C, N, O, S with the proviso that the X linking the ring to N is C.
er embodiment, the compounds have a structure
wherein a dashed line represents the presence or absence of a bond.
[00162] In another embodiment, the compounds have a structure
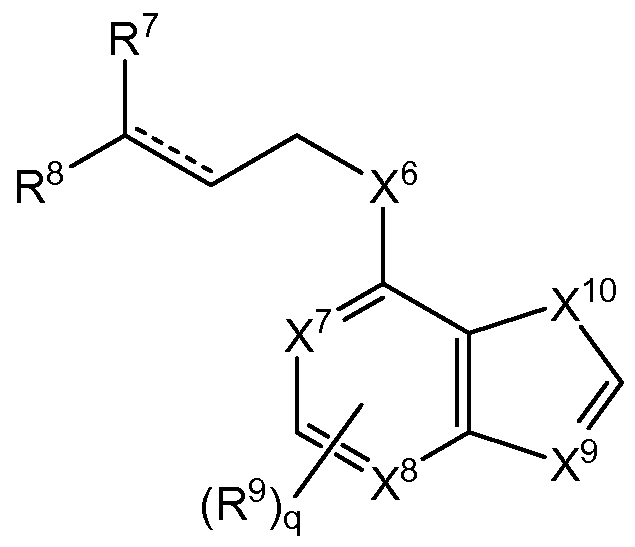
wherein a dashed line represents the presence or absence of a bond;
X8-X12 is each independently selected from C, N, O, S;
each R4 is independently H, OH, Ci-C6 alkyl, Ci-C6 alkylene, Ci-C6 alkynyl, halogen, cyano, Ci-C6 alkyloxy, aryl or heteroaryl each optionally substituted with a Ci-C6 alkyl, SH, NHR3, C02R3 or halogen;
R3 is H, OH, Ci-C6 alkyl, Ci-C6 alkylene, Ci-C6 alkynyl, halogen, carboxylic group, ester group, aldehyde or cyano;
p is 0 to 5; and
q is 0 to 6.
[00163] In other embodiments, the compounds have a structure
[00164] In still another embodiment, the compounds have a structure
[00165] Further still, compounds can have structures selected from
[00166] In one embodiment, R is OH.
[00167] In another embodiment, compounds have a structure selected from
[00168] In another embodiment, the compounds have a structure
wherein a dashed line represents the presence or absence of a bond.
[00169] In another embodiment, the compounds have a structure
wherein a dashed line represents the presence or absence of a bond;
Χδ-Χ is each independently selected from C, N, O, S;
each R4 is independently H, OH, Ci-C6 alkyl, Ci-C6 alkylene, Ci-C6 alkynyl, halogen, cyano, Ci-C6 alkyloxy, aryl or heteroaryl each optionally substituted with a Ci-C6 alkyl, SH, NHR3, C02R3 or halogen;
R3 is H, OH, Ci-C6 alkyl, Ci-C6 alkylene, Ci-C6 alkynyl, halogen, carboxylic group, ester group, aldehyde or cyano;
p is 0 to 5; and
q is 0 to 6.
[00170] In other embodiments, the compounds have a structure
[00171] In still another embodiment, the compounds have a structure
[00172] In one embodiment, the compound is meta-topolin, also known as 6-(3- hydroxybenzylamino)-purine, and by the abbreviation mT, having a empirical formula of C12H10N5OH, a molecular weight of 241.25, and the following structural formula:
[00173] wherein said meta-topolin is a derivative of a willow tree or a poplar tree.
[00174] Meta-topolin analogues particularly include, without limitation, meta-topolin riboside, meta-topolin-9-glucoside, ortho-topolin, ortho-topolin riboside, ortho-topolin-9- glucoside, para-topolin, para-topolin riboside, para-topolin-9-glucoside, ortho- methoxytopolin, ortho-methoxytopolin riboside, meta-methoxytopolin, meta-methoxytopolin riboside and meta-methoxytopolin-9-glucoside.
[00175] Compounds useful according to the present disclosure include thidiazuron analogues having a general formula
wherein V is an aryl or heteroaryl;
each R5 and R6 is each independently H, OH, Ci-C6 alkyl, Ci-C6 alkylene, Ci-C6 alkynyl, halogen, cyano, Ci-C6 alkyloxy, aryl or heteroaryl each optionally substituted with a Ci-C6 alkyl or halogen;
n is 0 to 4;
o is 0 to 5
X13-X16 is each independently selected from C, N, O, S;
Z1 and Z2 are each independently NH, O, SH or CH or Z1 and Z2 can be combined to form a substituted or unsubstituted aryl or heteroaryl; and
Y1 is O or S.
[00176] In another embodiment, compounds have a structure
v21
wherein X -X is each independently selected from C, N, O, S.
[00177] In other embodiments, compounds include
[00178] In one embodiment, the compound is thidiazuron, also known as l-phenyl-3- (l,2,3-thiadiazol-5-yl)urea and 5-phenylcarbamoylamino-l,2,3-thiadiazole, has the empirical formula of C9H8N4OS, a molecular weight of 220.25 and the following structural formula
[00179] Compounds useful according to the present disclosure include B-naphthoxy acetic analogues having a general formula:
or a salt thereof;
wherein Ra is COR3, C02R3, CONR3R4, or CN;
each Rb is independently R3; OR3; F; CI; Br; I; CN; N02; OCF3; CF3; NR2R3; SR3, SOR3, S02R3, C02R3, COR3, CONR3R4, CSNR4R5; or optionally substituted aryl or optionally substituted heteroaryl, wherein each substituent of aryl or heteroaryl is independently Ci_C6 alkyl, F, CI, Br, or I;
a is 1, 2, 3, 4, 5, 6, or 7;
Xa is NH, S or O;
each R3 is independently H, Ci_C6 alkyl, C2-C6 alkenyl, or C2-C6 alkynyl; and
each R4 is independently R3 or optionally substituted phenyl, wherein each substituent of phenyl is independently Ci_C6 alkyl, F, CI, Br, or I.
[00180] In another embodiment, compounds have a structure:
[00181] In one embodiment, the compound is B-naphthoxy acetic acid (NAA), also known as acetic acid, (2-naphthalenoxy)-(9CI) and has a CAS Number of 120-23-0, has the empirical formula of C12H10O3, a molecular weight of 202.21 and the following structural formula:
[00183] Compounds useful according to the present disclosure include indole butyric acid (IBA) analogues having a general formula:
or a salt thereof;
wherein R1 is COR3, C02R3, CONR3R4, or CN;
each R2 is independently R3; OR3; F; CI; Br; I; CN; N02; OCF3; CF3; NR2R3; SR3, SOR3, S02R3, C02R3, COR3, CONR3R4, CSNR4R5; or optionally substituted aryl or optionally substituted heteroaryl, wherein each substituent of aryl or heteroaryl is independently Ci_C6 alkyl, F, CI, Br, or I;
n is 1, 2, 3, or 4;
X is NH, S or O;
each R3 is independently H, Ci_C6 alkyl, C2_C6 alkenyl, or C2_C6 alkynyl; and
each R4 is independently R3 or optionally substituted phenyl, wherein each substituent of phenyl is independently Ci_C6 alkyl, F, CI, Br, or I.
[00184] In another embodiment, compounds have a structure:
[00185] In one embodiment, the compound is indole butyric acid (IBA), also known as 1- Indole-3-butanoic acid, and has a CAS Number of 133-32-4, has the empirical formula of Ci2Hi3N02, a molecular weight of 203.24, and the following structural formula:
[00186] Other examples of IBA analogues may include, but are not limited to:
wherein a dashed line represents the presence or absence of a bond;
each R5 and each R6 is independently R3; OR3; F; CI; Br; I; CN; N02; OCF3; CF3; NR2R3; SR3, SOR3, S02R3, C02R3, COR3, CONR3R4, CSNR4R5; or optionally substituted aryl or optionally substituted heteroaryl, wherein each substituent of aryl or heteroaryl is independently Ci_C6 alkyl, F, CI, Br, or I;
o is 0, 1, 2, 3, 4, or 5;
p is 0, 1, or 2;
X1 is NH, S or O;
X4 is -N= and X5 is -NH-, -S-, or -0-; or X5 is -N= and X4 is -NH-, -S-, or -0-;
X2 and X3 and are independently N or CR ;
each R3 is independently H, Ci_C6 alkyl, C2_C6 alkenyl, or C2_C6 alkynyl; and
each R4 is independently R3 or optionally substituted phenyl, wherein each substituent of phenyl is independently Ci_C6 alkyl, F, CI, Br, or I.
[00188] In some embodiments, X4 is -N= and X5 is -NH-, -S-, or -0-, and the dashed line represents the presence or absence of a bond. Thus, compounds of according to the formula below are contemplated.
[00189] In other embodiments, X
5 is -N= and X
4 is -NH-, -S-, or -0-. Thus, compounds of the formula below are contemplated.
[00190] In another embodiment, the compounds have a structure:
[00191] In another embodiment, the compounds have a structure
[00192] In one embodiment, the compound is benzylaminopurine (BAP), also known as 9H-Purin-6-amine, N-(phenylmethyl)-, which has a CAS Number of 1214-39-7, an empirical formula of C12HnN5, a molecular weight of 225.25, and the following structural formula:
[00193] Other examples of BAP analogues may include, but are not limited to:
[00194] Compounds useful according to the present disclosure include 6-y-y- (dimethylallylamino)-purine (2ip) analogues having a general formula:
wherein a dashed line represents the presence or absence of a bond;
wherein R7, R8, and each R9 are independently R3; OR3; F; CI; Br; I; CN; N02; OCF3; CF3; NR2R3; SR3, SOR3, S02R3, C02R3, COR3, CONR3R4, CSNR4R5; or optionally substituted aryl or optionally substituted heteroaryl, wherein each substituent of aryl or heteroaryl is independently Ci_C6 alkyl, F, CI, Br, or I;
q is 0, 1, or 2;
X6 is NH, S or O;
X9 is -N= and X10 is -NH-, -S-, or -0-; or X10 is -N= and X9 is -NH-, -S-, or -0-;
X7 and X8 and are independently N or CR9; and
each R3 is independently H, Ci_C6 alkyl, C2_C6 alkenyl, or C2_C6 alkynyl; and
each R4 is independently R3 or optionally substituted phenyl, wherein each substituent of phenyl is independently Ci_C6 alkyl, F, CI, Br, or I.
[00195] In some embodiments, the dashed line represents the presence or absence of a bond. Thus, compounds of according to the formulas below are contemplated.
[00196] In some embodiments X9 is -N= and X10 is -NH-, -S-, or -0-. Thus, compounds according the formula below are contemplated.
[00197] In other embodiments, X10 is -N= and X9 is -NH-, -S-, or -0-. Thus, compounds having the structure shown below are contemplated.
[00198] In another embodiment, compounds have a structure:
[00199] In one embodiment, the compound is 6-y,y,-(dimethylallylamino)-purine (2ip) or DAP, also known as 9H-purin-6-amine, N-(3-methyl-2-butene-l-yl)-, having a CAS No. 2365-40-4, an empirical formula of C10H13N5, a molecular weight of 203.24, and the following structural formula:
[00200] Other examples of 2ip analogues may include, but are not limited to:
[00201] Compounds useful according to the present disclosure include N,N-diphenylurea (DPU) analogues having a general formula:
or a salt thereof;
wherein each R10 and each R11 is independently R3; OR3; F; CI; Br; I; CN; N02; OCF3; CF3; NR2R3; SR3, SOR3, S02R3, C02R3, COR3, CONR3R4, CSNR4R5; or optionally substituted aryl or optionally substituted heteroaryl, wherein each substituent of aryl or heteroaryl is independently Ci_C6 alkyl, F, CI, Br, or I;
r and s are independently 0, 1 , 2, 3, 4, or 5;
X11 and X12 are independently NR10, S, or O;
each R3 is independently H, Ci_C6 alkyl, C2_C6 alkenyl, or C2_C6 alkynyl; and
each R4 is independently R3 or optionally substituted phenyl, wherein each substituent of phenyl is independently Ci_C6 alkyl, F, CI, Br, or I.
[00202] In another embodiment, compounds have a structure:
[00203] In one embodiment, the compound is Ν,Ν-diphenylurea (DPU), which is represented by a formula:
[00204] Other examples of DPU analogues may include, but are not limited to:
[00205] Compounds useful according to the present disclosure include N-pyridinyl-N'- phenylurea (PPU used interchangeably with CPPU herein) analogues having a general formula:
or a salt thereof;
wherein each R
12 and each R
13 is independently R
3; OR
3; F; CI; Br; I; CN; N0
2; OCF
3; CF
3; NR
2R
3; SR
3, SOR
3, S0
2R
3, C0
2R
3, COR
3, CONR
3R
4, CSNR
4R
5; or optionally substituted aryl or optionally substituted heteroaryl, wherein each substituent of aryl or heteroaryl is independently Ci_C
6 alkyl, F, CI, Br, or I;
t and u are independently 0, 1, 2, 3, 4, or 5;
X13 and X14 are independently NR12, S, or O;
each R3 is independently H, Ci_C6 alkyl, C2_C6 alkenyl, or C2_C6 alkynyl; and
each R4 is independently R3 or optionally substituted phenyl, wherein each substituent of phenyl is independently Ci_C6 alkyl, F, CI, Br, or I.
[00206] In one embodiment, compounds have a structure:
[00207] In another embodiment, compounds have a structure:
[00208] In one embodiment, the compound is N-(2-chloropyridin-4-yl)-N'-phi which is represented by a formula:
[00209] Other examples of PPU analogues may include, but are not limited to:
[00210] If present in a media, each cytokinin and/or auxin can be present in an amount from 0.001 mg/L- 100 mg/L and all amounts in between. In certain embodiments, meta-topolin and/or its analogues can be present at 0.001 mg/L, 0.01, 0.1, 1, 2, 3, 3.1, 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4, 4.1, 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9, 5, 5.1, 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9, 6, 6.1, 6.2, 6.3, 6.4, 6.5, 6.6, 6.7, 6.8, 6.9, 7, 7.1, 7.2, 7.3, 7.4, 7.5, 7.6, 7.7, 7.8, 7.9, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L. Meta-topolin and/or its analogues can be also be included in any amount up to 200 mg/L.
[00211] In particular embodiments, thidiazuron and/or its analogues can be present at 0.001 mg/L, 0.01, 0.025, 0.05, 0.075, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6, 0.65, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, 1.25, 1.50, 1.75, 2.0, 2.25, 2.5, 2.75, 3.5, 4.5, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L. Thidiazuron and/or its analogues can also be included in any amount up to 200 mg/L.
[00212] In particular embodiments, NAA and/or its analogues can be present at 0.001 mg/L, 0.01, 0.0125, 0.015, 0.0175, 0.02, 0.0225, 0.025, 0.0275, 0.03, 0.0325, 0.035, 0.0375, 0.04, 0.0425, 0.045, 0.0475, 0.05, 0.0525, 0.055, 0.0575, 0.06, 0.0625, 0.065, 0.0675, 0.07, 0.0725,
0.075, 0.0775, 0.08, 0.0825, 0.085, 0.0875, 0.09, 0.0925, 0.095, 0.0957, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6, 0.65, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, 1.25, 1.50, 1.75, 2.0, 2.25, 2.5, 2.75, 3.5, 4.5, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L. NAA and/or its analogues can be also be included any amount up to 200 mg/L.
[00213] In particular embodiments, IBA and/or its analogues can be present at 0.001 mg/L, 0.01, 0.025, 0.05, 0.075, 0.08, 0.1, 0.125, 0.15, 0.175, 0.2, 0.225, 0.25, 0.275, 0.3, 0.325, 0.35, 0.375, 0.4, 0.425, 0.45, 0.475, 0.5, 0.525, 0.55, 0.575, 0.6, 0.625, 0.65, 0.675, 0.7, 0.725, 0.75, 0.775, 0.8, 0.825, 0.85, 0.875, 0.9, 0.925, 0.95, 0.975, 1.0, 1.25, 1.50, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0, 3.25, 3.5, 3.75, 4.0, 4.5, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L. IBA and/or its analogues can be also be included any amount up to 200 mg/L.
[00214] In particular embodiments, BAP and/or its analogues can be present at 0.001 mg/L, 0.01, 0.025, 0.05, 0.06, 0.07, 0.0725, 0.075, 0.0775, 0.08, 0.0825, 0.085, 0.0875, 0.09, 0.0925, 0.095, 0.0975, 0.1, 0.125, 0.15, 0.175, 0.2, 0.225, 0.25, 0.275, 0.3, 0.325, 0.35, 0.375, 0.4, 0.425, 0.45, 0.475, 0.5, 0.525, 0.55, 0.575, 0.6, 0.625, 0.65, 0.675, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, 1.25, 1.50, 1.75, 2.0, 2.25, 2.5, 2.75, 3.5, 4.5, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L. BAP and/or its analogues can be also be included any amount up to 200 mg/L.
[00215] In particular embodiments, 2ip and/or its analogues can be present at 0.001 mg/L, 0.01, 0.025, 0.05, 0.075, 0.08, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6, 0.65, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, 1.25, 1.50, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, 4,0, 4.5, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82,
83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L.2ip and/or its analogues can be also be included any amount up to 200 mg/L.
[00216] In particular embodiments, DPU and/or its analogues can be present at 0.001 mg/L, 0.01, 0.025, 0.05, 0.075, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6, 0.65, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, 1.25, 1.50, 1.75, 2.0, 2.25, 2.5, 2.75, 3.5, 4.5, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L. DPU and/or its analogues can be also be included in any amount up to 200 mg/L.
[00217] In particular embodiments, CPPU and/or its analogues can be present at 0.001 mg/L, 0.01, 0.025, 0.05, 0.075, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6, 0.65, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, 1.25, 1.50, 1.75, 2.0, 2.25, 2.5, 2.75, 3.5, 4.5, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100 mg/L. CPPU and/or its analogues can be also be included in any amount up to 200 mg/L.
[00218] Cytokinins in combination with other cytokinins or auxins and auxins in combination with other auxins or cytokinins can also be utilized in ratios. For example, any two cytokinins and/or auxins in pairs disclosed herein can be included in the following exemplary ratios: 100:1, 95:1, 90:1, 85:1, 80:1, 75:1, 70:1, 65:1, 60:1, 55:1, 50:1, 45:1, 40:1, 35:1, 30:1, 29:1, 28:1, 27:1, 26:1, 25:1, 24:1, 23:1, 22:1, 21:1, 20:1, 19:1, 18:1, 17:1, 16:1, 15:1, 14:1, 13:1, 12:1, 11:1, 10:1; 9:1, 8:1, 7:1, 6.9:1, 6.8:1, 6.7:1, 6.6:1, 6.5:1, 6.4: 1, 6.3:1, 6.2:1, 6.1:1, 6:1, 5.9:1, 5.8:1, 5.7:1, 5.6:1, 5.5:1, 5.4:1, 5.3:1, 5.2:1, 5.1:1, 5:1; 4:1, 3:1, 2:1, 1:1, 0.75:1, 0.5:1, 0.25:1, 0.1:1, 0.075:1, 0.05:1, 0.025:1 or 0.001:1. These ratios can also be utilized between meta-topolin (and analogues) with thidiazuron (and analogues), with NAA (and analogues), with BAP (and analogues), with IBA (and analogues), with 2ip (and analogues), with DPU (and analogues) and/or with CPPU (and analogues). Similarly, the ratios can be utilized between thidiazuron (and analogues) with NAA (and analogues), with BAP (and analogues), with IBA (and analogues), with 2ip (and analogues), with DPU (and analogues) and/or with CPPU (and analogues). The ratios can also be utilized between NAA (and analogues) with BAP (and analogues), with IBA (and analogues), with 2ip (and analogues), with DPU (and analogues) and/or with CPPU (and analogues). The ratios can
also be utilized between BAP (and analogues) with IBA (and analogues), with 2ip (and analogues), with DPU (and analogues) and/or with CPPU (and analogues). In short, each of the cytokinins and/or auxins or its analogues can be included with a second cytokinin and/or auxin disclosed herein according to any of the disclosed ratios.
[00219] The structures or formula for a number of chemical compounds, including meta- topolin, thidiazuron, NAA, IBA, BAP, 2ip, DPU and CCPU and their analogues have been provided above. One of ordinary skill in the art will recognize reference to a compound should be construed broadly to include pharmaceutically acceptable salts, prodrugs, tautomers, alternate solid forms, non-covalent complexes, derivatives and combinations thereof, of a chemical entity of the depicted structure or chemical name.
[00220] A pharmaceutically acceptable salt is any salt of the parent compound that is suitable for use in the methods disclosed herein. A pharmaceutically acceptable salt also refers to any salt which may form as a result of administration of an acid, another salt, or a prodrug which is converted into an acid or salt. A salt comprises one or more ionic forms of the compound, such as a conjugate acid or base, associated with one or more corresponding counter-ions. Salts can form from or incorporate one or more deprotonated acidic groups (e.g. carboxylic acids), one or more protonated basic groups (e.g. amines), or both (e.g. zwitterions).
[00221] Not intended to be limited by the above described compounds, various tautomers of the above compounds may be possible. As used herein, "tautomer" refers to the migration of protons between adjacent single and double bonds. The tautomerization process is reversible. Other tautomers are possible when the compound includes, for example but not limited to, enol, keto, lactamin, amide, imidic acid, amine, and imine groups. Tautomers will generally reach an equilibrium state wherein the double bond is resonantly shared between the two bond lengths.
[00222] Unless stereochemistry is explicitly depicted, a structure is intended to include every possible stereoisomer, both pure or in any possible mixture.
[00223] Alternate solid forms are different solid forms than those that may result from practicing the procedures described herein. For example, alternate solid forms may be polymorphs, different kinds of amorphous solid forms, glasses, and the like.
[00224] Non-covalent complexes are complexes that may form between the compound and one or more additional chemical species that do not involve a covalent bonding interaction between the compound and the additional chemical species. They may or may not have a
specific ratio between the compound and the additional chemical species. Examples might include solvates, hydrates, charge transfer complexes, and the like.
[00225] As an overview, in typical micropropagation, plants are placed in various media that stimulate physiological processes such as growth and multiplication by and/or within the plant. Generally the process includes 3 steps (following explant preparation and disinfection, discussed below): (1) initiation of in vitro growth and/or multiplication of the explant in a media; (2) further in vitro multiplication in a media; and (3) transition to ex vitro conditions. Not every tissue culture process requires each step, however, and in certain processes, steps can be combined or skipped. For example, while there is commonly a change in media types between steps 1 and 2, in certain embodiments, a media change is not included. In other processes, plants may not require a particular step promoting transition to ex vitro conditions but instead complete the process in a same media that supports multiplication. Accordingly, as described herein, media are defined as Stage 1 media (1st media of a process); Stage 2 media (2nd media of a process); Stage 3 media (3rd media of a process); etc. Particular media can change stage based on the number of steps within a particular process and where the particular media resides within their order.
[00226] The number assigned to a media within a given process is maintained when a certain media is used more than one time. For example, certain embodiments disclosed herein include cycling explants or shoots in a rotation of media. For example, an explant may be placed in a Stage 1 media followed by a Stage 2 media and then returned back to its Stage 1 media. In this context, when exposure to a media is repeated, it retains its lowest Stage number within the particular process.
[00227] To begin the process, a Stage 1 media can be obtained or prepared. Stage 1 media include a pH that is generally hospitable to plants (typically from 4.0-7.0 or 4.5-6.5). Stage 1 media disclosed herein can include (i) meta-topolin; (2) at least three cytokinins; (3) the cytokinin meta-topolin or an analogue thereof in combination with at least two other cytokinins; (4) at least one auxin and at least two cytokinins; (5) at least two auxins and at least two cytokinins or (6) at least two auxins and at least three cytokinins. In particular embodiments, the cytokinins and auxins are chosen from one or more of meta-topolin, thidiazuron, NAA, IBA, BAP, 2ip, CCPU and DPU. In additional embodiments, the cytokinins and auxins are chosen from one or more of meta-topolin or analogues thereof, thidiazuron or analogues thereof, NAA or analogues thereof, IBA or analogues thereof, BAP or analogues thereof, 2ip or analogues thereof, CCPU or analogues thereof and DPU or analogues thereof.
[00228] One example of a Stage 1 media includes meta-topolin. Another non-limiting example includes meta-topolin, thidiazuron, NAA and BAP. Another non- limiting example includes meta-topolin, NAA and BAP. Another non- limiting example includes meta-topolin, NAA, BAP and IBA. Another non-limiting example includes meta-topolin, thidiazuron, NAA, BAP and IBA. Another non-limiting example includes thidiazuron, NAA, BAP and 2ip. Another non-limiting example includes thidiazuron, NAA and 2ip. Another non- limiting example includes meta-topolin, thidiazuron, NAA, BAP, IBA and 2ip. Another non-limiting example includes meta-topolin, IBA, 2ip and BAP. Another non- limiting example includes meta-topolin, thidiazuron, CPPU, NAA and BAP. Another non- limiting example includes meta-topolin, thidiazuron, DPU, NAA and BAP. Another non-limiting example includes thidiazuron, CPPU, BAP, IBA and 2ip. Another non- limiting example includes CPPU, DPU, NAA and BAP. Another non- limiting example includes meta-topolin, thidiazuron, CPPU, DPU, NAA, BAP, IBA and 2ip. Each of these non-limiting examples can also include analogues of meta-topolin, thidiazuron, NAA, BAP, DPU, CPPU, IBA and/or 2ip.
[00229] The Stage 1 media is then placed into test tubes or other appropriate containers (including jars, boxes, jugs, cups, sterile bag technology, bioreactors, temporary immersion vessels, etc. wherein when not specified are collectively referred to as "tubes"). These tubes can be capped or covered and autoclaved to sterilize the tubes and media. In another embodiment, sterilization is achieved by autoclaving at 5-25 pounds pressure psi at a temperature of 200° F - for 200° F 10-25 minutes. In another embodiment, sterilization is achieved by autoclaving at 15 pounds pressure psi at a temperature of 250° F for 15-18 minutes. Liquid media can be subjected to filter sterilization.
[00230] Sterility can also be assessed by an accepted number of contaminated tubes per hundred tubes, for example and without limitation, 0 contaminated tubes per hundred tubes, no more than 1 contaminated tube per hundred tubes, no more than 2 contaminated tubes per hundred tubes, no more than 3 contaminated tubes per hundred tubes, no more than 4 contaminated tubes per hundred tubes, no more than 5 contaminated tubes per hundred tubes, no more than 6 contaminated tubes per hundred tubes, no more than 7 contaminated tubes per hundred tubes, no more than 8 contaminated tubes per hundred tubes, no more than 9 contaminated tubes per hundred tubes, no more than 10 contaminated tubes per hundred tubes, etc.
[00231] In media containing a gelling agent, such as agar, agarose, gellan gum, carrageenan or combinations thereof, the media solidifies upon cooling and serves to provide the micropropagated plant tissues with support, nutrients, growth regulators, water and other compounds as described herein. Generally, tubes and jars contain 15-25 mL media while boxes contain 40-50 mL media. Cups can include 30-40 mL while jugs generally contain more than 50 mL. Sterile bag technology, bioreactors and temporary immersion bioreactors contain 50 mLs to 75,000 liters of media.
[00232] Micropropagated plants begin from a selected piece of plant tissue, called an "explant" or "mother plant." This explant is the source of cells to be developed during the tissue culturing process. The explant can be any segment or collection of cells from apical meristem, axillary buds, cambium, lateral meristem, shoot apices, stem segments, immature nodal sections from stems, lateral shoots, seeds, seedlings or leaf segments. In one embodiment, the explant is taken from a 1 year old bamboo plant. In another embodiment, the explant is taken from a 2 year old bamboo plant. In another embodiment, the explant is taken from a bamboo plant that is 5 years old or less. In another embodiment, the explant is taken from a bamboo plant that is 4 years old or less. In another embodiment, the explant is taken from a bamboo plant that is 3 years old or less. In another embodiment, the explant is taken from a bamboo plant that is 2 years old or less. In another embodiment, the explant is taken from a bamboo plant that is 1 year old or less. In another embodiment, the explant is taken from a bamboo plant that is 6 months old or less. In another embodiment, the explant is taken from a bamboo plant that is 3 months old or less. The bamboo from which the explant is obtained can be grown in any suitable husbandry situation, including but not limited to growing in a growth chamber, growing in a greenhouse or growing in a field. In one particular embodiment, growth in a greenhouse is excluded.
[00233] As will be understood by one of ordinary skill in the art, a variety of appropriate explants can be used in accordance with the present disclosure. In certain embodiments according to the present disclosure, immature nodal sections from stems can be used as the explant material. In one embodiment, the explants can be new growth canes with the lateral shoots just breaking the sheath at nodal section(s). New growth canes include those obtained from the plant within a current season or year, wherein such new growth canes can be obtained from any node on the plant. In one particular embodiment, explant material includes or is limited to the third node from the base of a cane.
[00234] Nodal section(s) can be cut into 3-5, 1-10, 2-9, 3-8, 4-6, 3-6 or 2-7 millimeter sections with the shoot intact and disinfected to remove pathogens on the exterior of the explant. Any disinfection method known in the art can be used. Exemplary disinfection methods include application of a disinfectant, such as a disinfectant selected from among bleach (sodium and/or potassium and/or calcium hypochlorite), alcohol (e.g., ethanol, isopropyl), ozone, chlorine gas, iodine solution, dichloroisocyanuric acid, dichloroisanuric acid, trichlorotriazinetriona, mercuric chloride, hydrogen peroxide, FungiGoneTM (bioWorld, Inc., Dublin, OH), plant preservatives, or antibiotic or anti-fungal solutions or combinations thereof, or subjecting the exposed surface of the explant to ultraviolet light or to a temperature of -20°C or lower or to a temperature higher than 40°C or 50°C for a short period of time. In certain embodiments, small amounts (a few drops) of Tween 20 can be added to the disinfecting solutions.
[00235] Following initial disinfection, the outer sheaths can be peeled off and discarded and the remaining piece put into an approximately 1%, 5%, 10%, 15%, 20%>, 25%, 30%, 35%, 40%, 45% or 50% solution of a commercial bleach or a similar disinfecting solution. In particular embodiments, the bleach can be heated to 20-60°C or 23-50°C. The peeled explant in disinfecting solution can be put onto a shaker table, such as for example, a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) for 10 minutes, 20 minutes, 30 minutes, 60 minutes, 90 minutes, 120 minutes, 150 minutes, 180 minutes, 210 minutes or up to 24, 36, or 48 hours at 6-9 or 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12, 13, 14, or 15 revolutions per minute. In another embodiment, the peeled explants can then be put into an approximately 1% solution of bleach or similar disinfecting solution, and placed back onto the shaker table for 30 minutes. In another embodiment, this 1% bleach or similar disinfecting solution step can be repeated. In another embodiment, these described steps are progressive and include the entire disinfection process. Sonication and vacuum infiltration of the tissue can also be used with the described disinfection procedures. As will be understood
by one of ordinary skill in the art, a variety of appropriate disinfecting procedures can be used in accordance with the present disclosure.
[00236] Once disinfected, the explants can be placed onto a Stage 1 media within the tube and the tubes can be placed in a regulated growth chamber. As used herein, "growth chambers" can include a number of configurations and sizes including table-top boxes, standalone chambers, closets, small rooms, large rooms, etc. As is understood by one of ordinary skill in the art, variables such as light or temperature can be appropriately controlled in such a growth chamber. Appropriate ranges for tissue culturing bamboo include from 65°F-70°F, 60°F-75°F or 55°F-80°F at 36-90, 25-100 or 15-130
Lighting can be full spectrum, although alternative lighting systems can also be utilized according to the present disclosure.
[00237] The explants are allowed to establish themselves within the tubes while in the growth chamber on Stage 1 media. In more common 3 stage tissue culturing, once established (i.e. growing without visible contamination), the cell cultures grown from the explants are transferred into a second, Stage 2 media. Alternatively, once established, the cell cultures can remain in Stage 1 media. At this stage in the tissue culturing process, a large number of plants can be created within a relatively short period of time because each cell culture can develop multiple shoots and each shoot can be separated and placed into an individual tube where it will develop additional shoots to separate and multiply.
[00238] Stage 2 media disclosed herein can include (i) meta-topolin; (2) at least three cytokinins; (3) the cytokinin meta-topolin or an analogue thereof in combination with at least two other cytokinins; (4) at least one auxin and at least two cytokinins; (5) at least two auxins and at least two cytokinins or (6) at least two auxins and at least three cytokinins. In particular embodiments, the cytokinins and auxins are chosen from one or more of meta- topolin, thidiazuron, NAA, IBA, BAP, 2ip, CCPU and DPU. In additional embodiments, the cytokinins and auxins are chosen from one or more of meta-topolin or analogues thereof, thidiazuron or analogues thereof, NAA or analogues thereof, IBA or analogues thereof, BAP or analogues thereof, 2ip or analogues thereof, CCPU or analogues thereof and DPU or analogues thereof.
[00239] One example of a Stage 2 media includes meta-topolin. Another non-limiting example includes meta-topolin, thidiazuron, NAA and BAP. Another non- limiting example includes meta-topolin, NAA and BAP. Another non- limiting example includes meta-topolin, NAA, BAP and IBA. Another non-limiting example includes meta-topolin, thidiazuron, NAA, BAP and IBA. Another non-limiting example includes thidiazuron, NAA, BAP and
2ip. Another non-limiting example includes thidiazuron, NAA and 2ip. Another non- limiting example includes meta-topolin, thidiazuron, NAA, BAP, IBA and 2ip. Another non-limiting example includes meta-topolin, IBA, 2ip and BAP. Another non- limiting example includes meta-topolin, thidiazuron, CPPU, NAA and BAP. Another non- limiting example includes meta-topolin, thidiazuron, DPU, NAA and BAP. Another non-limiting example includes thidiazuron, CPPU, BAP, IBA and 2ip. Another non- limiting example includes CPPU, DPU, NAA and BAP. Another non- limiting example includes meta-topolin, thidiazuron, CPPU, DPU, NAA, BAP, IBA and 2ip. Each of these non-limiting examples can also include analogues of meta-topolin, thidiazuron, NAA, BAP, DPU, CPPU, IBA and/or 2ip.
[00240] Without limiting the media to a particular stage, non- limiting examples of media that commonly serve as Stage 1 and/or Stage 2 media include (all amounts mg/L unless otherwise noted):
Media b-12c(
Media CW3(i-v):
Component Standard Standard Standard Standard Standard CW3-i CW3-ii CW3-iii CW3-iv CW3-v
Thiamine 0.2-0.6 0.3-0.5 0.36-0.44 0.4 0.4±0.2
NAA 0.05-0.15 0.07-0.12 0.09-0.1 1 0.1 0.1±0.02
BAP 0.5 - 1.5 0.7 - 1.3 0.9-1.1 1 1±0.2
IBA 0.1-0.3 0.15-0.25 0.17-0.22 0.2 0.2±0.1
Meta-topolin 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.7-8.2 4.1-6.8 4.9-6.1 5.5 5.5±0.2
Media b-9(i-v)
Media CW4(i- v):
Component Standard Standard Standard Standard Standard
CW4-i CW4-ii CW4-iii CW4-iv CW4-v
NH4N03 825-2475 1237-2063 1485-1815 1650 1650±2
KNO3 950-2850 1425-2375 1710-2090 1900 1900±2
MgS04 185-555 275-465 330-410 370 370±2
MnS04 8-26 12-22 15 -19 16.9 16.9±0.2
ZnS04 4-12 6-10 8-9 8.6 8.6±0.2
CuS04 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
Media CWl(i-v):
Component Standard Standard Standard Standard Standard
CW5-i CW5-ii CW5-iii CW5-iv CW5-v
Thiamine 0.2-0.6 0.3-0.5 0.36-0.44 0.4 0.4±0.2
NAA 0.02 - 0.08 0.03-0.07 0.04-0.06 0.05 0.05±.02
BAP 0.5 - 1.5 0.7 - 1.3 0.9-1.1 1 1±0.2
IBA 0.1-0.3 0.15-0.25 0.17-0.22 0.2 0.2±0.1
Meta-topolin 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.7-8.2 4.1-6.8 4.9-6.1 5.5 5.5±0.2
Silicon 0.5 - 1.5 0.7 - 1.3 0.9-1.1 1 1±0.2
Solution mL
Media CW6(i- v):
Component Standard Standard Standard Standard Standard
CW6-1 CW6-ii CW6-iii CW6-1V CW6-v
NH4NO3 825-2475 1237-2063 1485-1815 1650 1650±2
KNO3 950-2850 1425-2375 1710-2090 1900 1900±2
MgS04 185-555 275-465 330-410 370 370±2
MnS04 8-26 12-22 15-19 16.9 16.9±0.2
ZnS04 4-12 6-10 8-9 8.6 8.6±0.2
CuS04 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
CaCl2 220-660 330-350 400-480 440 440±2
Kl 0.4-1.2 0.6-1.0 0.75-0.90 0.83 0.83±.02
CoCl2 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
H3BO3 3-9 4-8 5-7 6.2 6.2±0.2
Na2Mo04 0.12-0.36 0.18-0.31 .22-.28 0.25 0.25±.02
KH2P04 85-255 120-210 150-190 170 170±2
K2S05 181.8-545.6 272.8-454.6 327.4-400.0 363.75 363.75±.02
FeS04 27-84 40-70 50-60 55.7 55.7±0.2
Na2EDTA 37-112 55-94 67-82 74.6 74.6±0.2
Na2H2P04 85-255 120-210 150-190 170 170±2 myo-Inositol 50-150 75-125 90-110 100 100±2
Thiamine 0.2-0.6 0.3-0.5 0.36-0.44 0.4 0.4±0.2
NAA 0.05-0.15 0.07-0.12 0.09-0.11 0.1 0.1±0.02
BAP 0.5 - 1.5 0.7 - 1.3 0.9-1.1 1 1±0.2
IBA 0.1-0.3 0.15-0.25 0.17-0.22 0.2 0.2±0.1
Meta-topolin 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
Thidiazuron 0.12-0.36 0.18-0.31 .22-.28 0.25 0.25±.02
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.7-8.2 4.1-6.8 4.9-6.1 5.5 5.5±0.2
Media b-lO(i-v):
Component Standard Standard Standard Standard Standard b-10-i b-10-ii b-10-iii b-10-iv b-10-v
NH4N03 825-2475 1237-2063 1485-1815 1650 1650±2
Component Standard Standard Standard Standard Standard b-10-i b-10-ii b-10-iii b-10-iv b-10-v
KNO3 950-2850 1425-2375 1710-2090 1900 1900±2
MgS04 185-555 275-465 330-410 370 370±2
MnS04 8-26 12-22 15-19 16.9 16.9±0.2
ZnS04 4.0-12.0 6.0-10.0 8.0-9.0 8.6 8.6±0.2
CuS04 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
CaCl2 220-660 330-350 400-480 440 440±2
Kl 0.40-1.25 0.60-1.05 0.75-0.90 0.83 0.83±.02
CoCl2 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
H3BO3 3-9 4-8 5-7 6.2 6.2±0.2
Na2Mo04 0.12-0.36 0.18-0.31 .22-.28 0.25 0.25±.02
KH2P04 85-255 120-210 150-190 170 170±2
FeS04 27-84 40-70 50-60 55.7 55.7±0.2
Na2EDTA 37.-112 55-94 67-82 74.6 74.6±0.2
Na2H2P04 85-255 120-210 150-190 170 170±2 myo-Inositol 50-150 75-125 90-110 100 100±2
Thiamine 0.2-0.6 0.3-0.5 0.36-0.44 0.4 0.4±0.2
NAA 0.02 - 0.08 0.03-0.07 0.04-0.06 0.05 0.05±.02
BAP 0.5 - 1.5 0.7 - 1.3 0.9-1.1 1 1±0.2
Meta-topolin 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
Media b-l l(i-v):
Component Standard Standard Standard Standard Standard b-ll-i b-ll-ii b-ll-iii b-ll-iv b-ll-v
Meta-topolin 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.7-8.2 4.1-6.8 4.9-6.1 5.5 5.5±0.2
Media b-l(i-v):
Media b-4(i-v):
Component Standard Standard Standard Standard Standard b-4-i b-4-ii b-4-iii b-4-iv b-4-v
NH4N03 600-1800 900-1500 1080-1320 1200 1200±2
Ca(N03)2 838-2515 1257-2096 1510-1844 1677 1677±2
K2S04 121-363 181-302 218-266 242 242±2
MgS04 270-830 410-690 500-610 555 555±2
MnS04 12.6-38.0 19.0-31.7 22.8-27.8 25.35 25.35±.02
ZnS04 6.4-19.5 9.6-16.2 11.5-14.0 12.9 12.9±0.2
CuS04 0.01-0.05 0.02-0.04 0.03-0.04 0.037 0.037±.002
Media b-6(i-v):
Component Standard Standard Standard Standard Standard b-6-i b-6-ii b-6-iii b-6-iv b-6-v
NH4N03 600-1800 900-1500 1080-1320 1200 1200±2
Ca(N03)2 838-2515 1257-2096 1510-1844 1677 1677±2
K2S04 121-363 181-302 218-266 242 242±2
MgS04 270-830 410-690 500-610 555 555±2
MnS04 12.6-38.0 19.0-31.7 22.8-27.8 25.35 25.35±.02
ZnS04 6.4-19.5 9.6-16.2 11.5-14.0 12.9 12.9±0.2
CuS04 0.01-0.05 0.02-0.04 0.033-0.041 0.037 0.037±.002
CaCl2 48-144 72-120 85-105 96 96±2
H3B03 3-9 4-8 5-7 6.2 6.2±0.2
Na2Mo04 0.12-0.36 0.18-0.31 .22-.28 0.25 0.25±.02
KH2P04 85-255 120-210 150-190 170 170±2
FeS04 27-84 40-70 50 -60 55.7 55.7±0.2
Na2EDTA 37-112 55-94 67-82 74.6 74.6±0.2
Na2H2P04 42-128 63-106 75-95 85 85±2 myo-Inositol 100-300 150-250 180-220 200 200±2
Thiamine 0.4-1.4 0.6-1.1 0.8-1.0 0.9 0.9±0.2
Pyridoxine 0.2-0.8 0.3-0.7 0.4-0.6 0.5 0.5±0.2
Nicotinic acid 0.2-0.8 0.3-0.7 0.4-0.6 0.5 0.5±0.2
Glycine 1-3 1.5-2.5 1.75-2.25 2 2±1
Riboflavin 10-30 15-25 18-22 20 20±2
NAA 0.5 - 1.5 0.7 - 1.3 0.9-1.1 1 1±0.2
Media B-9N2
Component Standard Standard Standard Standard Standard
Β-9Ν2-Ϊ B-9N2-ii B-9N2-iii B-9N2-iv B-9N2-V
NH4NO3 825-2475 1237-2063 1485-1815 1650 1650±2
KNO3 950-2850 1425-2375 1710-2090 1900 1900±2
K2S04 242-726 363-605 436-532 484 484±2
MgS04 185-555 275-465 330-410 370 370±2
MnS04 8-26 12-22 15-19 16.9 16.9±0.2
ZnS04 4-12 6-10 8-9 8.6 8.6±0.2
CuS04 0.01-0.37 0.02-0.03 0.02-0.028 0.025 0.025±.002
CaCl2 220-660 330-350 400-480 440 440±2
KI 0.40-1.25 0.60-1.05 0.75-0.90 0.83 0.83±.02
CoCl2 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
H3BO3 3.0-9.0 4.0-8.0 5.0-7.0 6.2 6.2±0.2
Na2Mo04 0.12-0.36 0.18-0.31 .22-.28 0.25 0.25±.02
KH2P04 85-255 120-210 150-190 170 170±2
FeS04 27-84 40-70 50-60 55.7 55.7±0.2
Na2EDTA 37-112 55-94 67-82 74.6 74.6±0.2
Na2H2P04 85-255 120-210 150-190 170 170±2 myo-Inositol 100-300 150-250 180-220 200 200±2
Thiamine 0.45-1.35 0.67-1.13 0.8-1.0 0.9 0.9±0.2
NAA 0.15-0.45 0.22-0.38 0.27-0.33 0.3 0.3±0.2
BAP 1-3 1.25-2.75 1.5-2.5 2 2±0.5
Thidiazuron 0.25-0.75 0.37-0.63 0.45-0.55 0.5 0.5±.2
Meta-Topolin 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.7-8.2 4.1-6.8 4.9-6.1 5.5 5.5±0.2
Media B-12C CPPU
Component Standard Standard Standard Standard Standard
B-12C B-12C B-12C B-12C B-12C
CPPU-i CPPU-ii CPPU-iii CPPU-iv CPPU-v
NH4N03 825-2475 1237-2063 1485-1815 1650 1650±2
K O3 950-2850 1425-2375 1710-2090 1900 1900±2
Ca(N03)2 225-775 410-690 495-605 550 550±2
MgS04 185-555 275-465 330-410 370 370±2
MnS04 8-26 12-22 15-19 16.9 16.9±0.2
ZnS04 4-12 6-10 8-9 8.6 8.6±0.2
CuS04 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
CaCl2 220-660 330-350 400-480 440 440±2
KI 0.40-1.25 0.60-1.05 0.75-0.90 0.83 0.83±.02
CoCl2 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
H3BO3 3-9 4-8 5-7 6.2 6.2±0.2
Na2Mo04 0.12-0.36 0.18-0.31 .22-.28 0.25 0.25±.02
KH2P04 85-255 120-210 150-190 170 170±2
FeS04 27-84 40-70 50-60 55.7 55.7±0.2
Na2EDTA 37-112 55-94 67-82 74.6 74.6±0.2
Na2H2P04 85-255 120-210 150-190 170 170±2 myo-Inositol 50-150 75-125 90-110 100 100±2
Thiamine 0.2-0.6 0.3-0.5 0.36-0.44 0.4 0.4±0.2
Casein 1-3 1.5-2.5 1.75-2.25 2 2±1
Hydroxylate g/L
NAA 0.02 - 0.08 0.03-0.07 0.04-0.06 0.05 0.05±.02
BAP 0.5 - 1.5 0.7 - 1.3 0.9-1.1 1 1±0.2
Thidiazuron 0.36 - 1.12 0.56-0.94 0.67-.083 0.75 0.75±.02
Meta-Topolin 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
CPPU 0.36 - 1.12 0.56-0.94 0.67-.083 0.75 0.75±.02
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.7-8.2 4.1-6.8 4.9-6.1 5.5 5.5±0.2
Media B-12C DPU
Component Standard Standard Standard Standard Standard
B-12C B-12C B-12C B-12C B-12C
CPU-i CPU-ii CPU-iii CPU-iv CPU-v
NH4N03 825-2475 1237-2063 1485-1815 1650 1650±2
KN03 950-2850 1425-2375 1710-2090 1900 1900±2
Ca(N03)2 225-775 410-690 495-605 550 550±2
MgS04 185-555 275-465 330-410 370 370±2
MnS04 8-26 12-22 15-19 16.9 16.9±0.2
ZnS04 4-12 6-10 8-9 8.6 8.6±0.2
CuS04 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
CaCl2 220-660 330-350 400-480 440 440±2
KI 0.40-1.25 0.60-1.05 0.75-0.90 0.83 0.83±.02
CoCl2 0.01-0.37 0.02-0.03 0.022-0.028 0.025 0.025±.002
H3B03 3-9 4-8 5-7 6.2 6.2±0.2
Na2Mo04 0.12-0.36 0.18-0.31 .22-.28 0.25 0.25±.02
KH2P04 85-255 120-210 150-190 170 170±2
FeS04 27-84 40-70 50-60 55.7 55.7±0.2
Na2EDTA 37-112 55-94 67-82 74.6 74.6±0.2
[0102] Note that for each of these media, its solid form is provided. Each media can be transformed into a liquid media by removing agar or carageenan and liquid forms of these media and their uses are expressly included within the scope of the present disclosure.
[0103] Media disclosed herein also include those described above (and below as spiked or reduced versions), wherein the non-cytokinin components are those found in Anderson's Rhododendron, Chu's N-6, DKW, Gamborg's B-5, Hoaglands No. 2, Kao & Michayluk, Nitsch & Nitsch, Schenk and Hildebrant, Vacin and Went, Whites and WPM, available from commercial sources such as Caisson Laboratories, Inc.. Particular media can have higher or lower levels of macronutrients than those provided in the preceding tables and others will lack nitrates. Particular embodiments have higher or lower levels of macronutrients and lack nitrates. More particular embodiments have higher levels of macronutrients and lack nitrates.
[0104] Media disclosed herein also include spiked media. Spiked media are those in which the concentration of at least one cytokinin and/or auxin in the media described above is increased by, for example and without limitation, 1%, 3%, 5%, 7%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90% 95%, 100%, 105%, 110% or 200%. In other embodiments, the concentration of at least one cytokinin and/or auxin in the media described above is increased by, for example and without limitation, 1-10%, 5-15%, 10-20%, 15-25%, 20-30%, 25-35%, 30-40%, 35-45%, 40-50%, 45-55%, 50-60%, 55-65%, 60-70%, 65-75%, 70-80%, 75-85%, 80-90%, 85-95%, 90-100%, 95-105%, 100-110%, 105-115%, 110-120%, 115-125%, 120-130%, 125-135%, 130-140%, 135-145%, 140-150%, 145-155%, 150-160%, 155-165%, 160-170%, 165-175%, 170-180%,
175-185%, 180-190%, 185-195%, 190-200%, 195-205%, 3-6%, 7-17%, 12-22%, 17-27%, 22-32%, 27-37%, 32-42%, 37-47%, 42-52%, 47-57%, 52-62%, 57-67%, 62-72%, 67-77%, 72-82%, 77-87%, 82-92%, 87-97%, 92-102%, 97-107%, 102-112%, 107-117%, 112-122%, 117-127%, 122-132%, 127-137%, 132-142%, 137-147%, 142-152%, 147-157%, 152-162%, 157-167%, 162-172%, 167-177%, 172-182%, 177-187%, 162-172%, 167-177%, 182-192%, 187-197%, 192-202%, 197-207%, or 200-210%. When more than one cytokinin and/or auxin is spiked, the concentrations of each raised cytokinin and/or auxin can be raised by the same amount or a different amount than other cytokinins and/or auxins in the media.
[0105] The following tables provide non- limiting examples of spiked media disclosed herein. Each media includes the components described in its respective table above or adjusted as described in paragraph [00108] with the following adjustments to cytokinin levels.
Media b-12c(
Component Spiked Spiked Spiked Spiked Spiked b-9-i b-9-ii b-9-iii b-9-iv b-9-v
Thidiazuron 0.5 1 1.5 1.75 0.25
Meta-topolin 9 8 7 6 500
Media CW4(i- v):
Component Spiked Spiked Spiked Spiked Spiked
CW4-i CW4-H CW4-iii CW4-iv CW4-v
NAA 0.1 0.1 0.1 0.1 0.1
BAP 1 1 2 3 4
IBA 0.4 0.2 1 0.5 0.2
Thidiazuron 0.25 0.5 1 5 0.3
Meta-topolin 50 40 5 15 10
Media CW1( v):
Component Spiked Spiked Spiked Spiked Spiked CW1-
CWl-i CWl-ii CWl-iii CWl-iv V
NAA 2 0.05 0.05 10 0.15
BAP 1 100 150 80 5
Meta-topolin 85 300 100 5 25
Media CW5(i- v):
Component Spiked Spiked Spiked Spiked Spiked CW5-
CW5-i CW5-ii CW5-iii CW5-iv V
NAA 0.05 0.15 0.05 0.1 3
BAP 1 1 5 1 4
IBA 0.2 0.4 0.2 1 5
Meta-topolin 1000 890 75 30 5
Media CW6(i- v):
Component Spiked Spiked Spiked Spiked Spiked CW6-
CW6-i CW6-ii CW6-iii CW6-iv V
NAA 0.1 0.1 2 0.4 0.5
BAP 3 1 4 1.5 2
IBA 0.5 0.2 0.4 0.3 1
Thidiazuron 0.25 0.25 0.5 10 3
Meta-topolin 450 20 10 5 7.5
Media b-lO(i-v):
Component Spiked Spiked Spiked Spiked Spiked
b-10-i b-10-ii b-10-iii b-10-iv b-10-v
NAA 0.05 0.05 1 0.25 0.05
BAP 1.3 3 1.6 2 1
Meta-topolin 65 70 75 80 20
Media b-ll(i-v):
CPPU-i CPPU-ii CPPU-iii CPPU-iv CPPU-v
NAA 0.75 1 0.05 1 0.05
BAP 1.25 2 1 1 2
Thidiazuron 1 1.5 1 0.75 1.5
Meta-Topolin 6 10 8 5 5
CPPU 0.8 1.5 1.5 0.75 0.75
Media B-12C DPU
Component Spiked Spiked Spiked Spiked Spiked
B-12C B-12C CPU- B-12C B-12C B-12C
CPU-i ii CPU-iii CPU-iv CPU-v
NAA 0.1 0.1 1.8 3 0.3
BAP 1.5 2 1.8 2 1.5
Thidiazuron 1.5 1.5 1.8 17 3
Meta-Topolin 10 9 5 6 5
DPU 4 3 1.8 0.75 0.75
[0106] Additional spiked media can include any standard media described above with the addition or adjustment to the following cytokinin and/or auxin concentrations:
Media AA
Component AA-i AA-ii AA-iii
BAP 1 1 1
Thidiazuron 2.5 5 10
Media AB
Component AB-i AB-ii AB-iii AB-iv
BAP 5 10 20 40
Media AC
Component AC-i AC-ii AC-iii AC-iv AC-v
BAP 1 1 1 1 1
CPPU 2.5 7.5 10 25 50
[00241] As explained more fully below, when a spiked media is utilized, explants or shoots generally remain on the spiked media for a shorter period of time than those kept on non- spiked media and following culture on a spiked media, the explants or shoots are transferred to a media containing standard, reduced or no levels of cytokinins and/or auxins (those containing reduced or no cytokinins and/or auxins are both referred to as "reduced" media herein).
[00242] The expected number of shoots may be different at different stages of the tissue culturing process and can also depend on the species of bamboo. In general, however, at the beginning of the process, multiplication is from 1.0-2.0, 1.0-3.0 or 2.0-3.0 times. Once established, multiplication can depend on the chosen container. For example, multiplication can range from, without limitation, 1-10 or 2-6 plants per tube, 1-15 or 4-9 plants per jar, 1- 20 or 9-17 plants per box or 1-50 or 20-35 plants per jug, 1-100,000 for sterile bag technology, bioreactors and temporary immersion bioreactors. The number 1 is included because certain species or particular cell cultures require more time in Stage 1 or Stage 2 media before multiplication begins. By carrying them through the process, however, most if not all begin multiplication within a number of cycles. For example, some cell cultures may begin to multiply only after 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 or 18 months in culture.
[00243] Methods disclosed herein can produce the following non-limiting number of shoots from a single explant: 100, 500, 1,000; 5,000; 10,000, 20,000, 50,000, 100,000, 250,000, 500,000, 750,000, 1,000,000 or more.
[00244] Following multiplication through culturing and subculturing, particular plant shoots can be selected for transition to ex vitro conditions. Generally, media that support transition to ex vitro conditions represent a Stage 2, Stage 3, Stage 4, Stage 5, Stage 6 media, Stage 7 media or Stage 8 media. Non- limiting examples of such media include:
Media Ech(i-v):
Component Standard Standard Standard Standard Standard
Ech-i Ech-ii Ech-iii Ech-iv Ech-v
Nicotinic 0.2-0.8 0.3-0.7 0.4-0.6 0.5 0.5±0.2 acid
Glycine 1-3 1.5-2.5 1.75-2.25 2 2±1
NAA 0.05-0.15 0.07-0.12 0.09-0.11 0.1 0.1±0.02
IAA 0.02 - 0.08 0.03-0.07 0.04-0.06 0.05 0.05±.02
Sugar g/L 15-45 22-37 27-33 30 30±2
Agar g/L 2.7-8.2 4.1-6.8 4.9-6.1 5.5 5.5±0.2
Media BR-2(i-v):
Media Amel(i-v):
Charcoal g/L 2.5-7.5 3.7-6.2 4.5-5.5 5 5±2
[00245] Non-limiting examples of spiked versions of these media include:
Media Ech(i-v):
[00246] During transition to ex vitro conditions, shoots and media can be placed in air permeable or air impermeable containers.
[00247] Each of the media described herein can be used in combination with each other media in a method, system or kit described herein. Moreover, the media can be combined in combinations greater than two (e.g., a kit may include 2 of the different media provided herein, or include 3 of the different media provided herein, or include more than 3 of the different media described herein). While not explicitly describing each possible combination herein, one of ordinary skill in the art should understand that this disclosure supports all possible combinations.
[00248] Following transition to ex vitro conditions, but before plants are placed in soil, or exposed to less regulated growing conditions, the plants can undergo a series of treatments designed to acclimate them to an unregulated growing environment. This is because some plants, when microcultured, do not develop adequate defensive structures, such as waxy cuticles to protect them from ordinary environmental conditions. The treatments that plants can undergo prior to being placed in an unregulated environment can include, without limitation, acclimatization to humidity, acclimatization to variations in temperature, and acclimatization to wind pressure. These acclimatization factors can be introduced gradually and/or in a staggered fashion.
[00249] Representative genus of bamboo appropriate for use with the disclosures herein include: Acidosasa; Ampelocalamus; Arundinaria; Bambusa; Bashania; Borinda;
Brachystachyum; Cephalostachyum; Chimonobambusa; Chimonocalamus; Chusquea;
Dendrocalamus; Dinochloa; Drepanostachyum; Eremitis; Fargesia; Gaoligongshania;
Gigantochloa; Guadua; Hibanobambusa; Himalayacalamus; Indocalamus; Indosasa;
Lithachne; Melocalamus; Melocanna; Menstruocalamus; Nastus; Neohouzeaua; Neololeba; Ochlandra; Oligostachyum; Olmeca; Otatea; Oxytenanthera; Phyllostachys; Pleioblastus;
Pseudosasa; Raddia; Rhipidocladum; Sarocalamus; Sasa; Sasaella; Sasamorpha;
Schizostachyum; Semiarundinaria; Shibataea; Sinobambusa; Thamnocalamus;
Thyrsostachys; and Yushania.
[00250] Non-limiting examples of species within these genus include:
[00251] Acidosasa: Edulis
[00252] Ampelocalamus: Scandens
[00253] Arundinaria: Arundinaria appalachiana; Arundinaria funghomii; Arundinaria gigantea; Arundinaria gigantea 'Macon'; and Arundinaria Tecta
[00254] Bambusa: arnhemica; balcooa; bambos; basihirsuta; beecheyana; beecheyana var pubescens; blumeana; boniopsis; burmanica; chungii; chungii var. Barbelatta; cornigera; dissimulator; dissimulator 'Albinodia'; distegia; dolichoclada; dolichoclada 'Stripe'; dolichomerithalla 'Green stripe'; dolichomerithalla 'Silverstripe'; emeiensis 'Chrysotrichus'; emeiensis 'Flavidovirens'; emeiensis 'Viridiflavus'; eutuldoides; eutuldoides 'Viridivittata'; gibba; glaucophylla; intermedia; lako; lapidea; longispiculata; maculata; malingensis; multiplex; multiplex lphonse Karr'; multiplex 'Fernleaf Stripestem'; multiplex 'Fernleaf ; multiplex 'Golden Goddess'; multiplex 'Goldstripe'; multiplex 'Midori Green'; multiplex
'Riviereorum'; multiplex 'Silverstripe'; multiplex 'Tiny Fern Striped'; multiplex 'Tiny Fern'; multiplex 'Willowy'; nutans; odashimae; odashimae X B. Tuldoides; oldhamii; oliveriana; pachinensis; pervariabilis; pervariabilis 'Viridistriatus'; rigida; rutila; sinospinosa; sp 'Hirose'; sp. 'Clone X'; sp. 'Nana'; sp. 'Polymorpha'; sp. 'Richard Waldron'; stenostachya; suberecta; textilis; textilis 'Dwarf; textilis 'Kanapaha'; textilis 'Maculata'; textilis 'Mutabilis'; textilis
'Scranton'; textilis var. Albostriata; textilis var. Glabra; textilis var. Gracilis; tulda; tulda 'Striata'; Tuldoides; variostriata; ventricosa; ventricosa 'Kimmei'; vulgaris; vulgaris 'Vittata'; vulgaris 'Wamin Striata'; and vulgaris 'Wamin'
[00255] Bashania: Fargesii; and Qingchengshanensis
[00256] Borinda: KR 5288; Albocerea; Angustissima; Contracta; Frigidorum; Fungosa; fungosa 'White Cloud'; Lushuiensis; Macclureana; Nujiangensis; Papyrifera; Perlonga; sp. 'Muliensis'; and Yulongshanensis
[00257] Brachystachyum: densifiorum; and densifiorum var. villosum
[00258] Cephalostachyum: Pergracile; and Virgatum
[00259] Chimonobambusa: macrophylla 'Intermedia'; Marmorea; marmorea 'Variegata'; Quadrangularis; quadrangularis 'Joseph de Jussieu'; quadrangularis 'Suow'; quadrangularis 'Yellow Groove'; Szechuanensis; and Tumidissinoda
[00260] Chimonocalamus: Pallens
[00261] Chusquea: Andina; Circinata; circinata 'Chiapas' Coronalis; Culeou; culeou Argentina'; culeou 'Cana Prieta'; culeou 'Hillier's Form'; Cumingii; Delicatula; Foliosa; Galeottiana; Gigantea; Glauca; Liebmannii; Macrostachya; mimosa ssp. Australis; Montana; Muelleri; Pittieri; Simplicifiora; sp. 'Chiconquiaco'; sp. 'Las Vigas'; Subtilis; Sulcata; Tomentosa; Uliginosa; Valdiviensis; and Virgata
[00262] Dendrocalamus: Asper; asper 'Betung Hitam'; Brandisii; brandisii 'Black'; brandisii (variegated); Calostachyus; Giganteus; giganteus (Quail Clone); giganteus (variegated); Hamiltonii; Jianshuiensis; jianshuiensis (variegated); Latiflorus; latiflorus 'Mei-nung';
Membranaceus; Minor; minor 'Amoenus'; Sikkimensis; Sinicus; sp. 'Maroochy'; sp. 'Parker's Giant'; Strictus; Validus; and Yunnanicus
[00263] Dinochloa: Malayana; and Scandens
[00264] Drepanostachyum: falcatum var. sengteeanum; and Khasianum
[00265] Eremitis: Eremitis
[00266] Fargesia: Adpressa; Apircirubens; apircirubens 'White Dragon'; Denudata; dracocephala 'Rufa'; Murieliae; murieliae 'SABE 939'; murieliae 'Vampire'; murieliae (next generation seedlings); Nitida; nitida 'Jiuzhaigou'; Robusta; robusta 'Campbell'; robusta 'Wolong'; sp. 'Scabrida'; and Utilis
[00267] Gaoligongshania: Gaoligongshania and Megalothyrsa
[00268] Gigantochloa: Hitam Hijau; Albociliata; Apus; Atroviolacea; Atter; Hasskarliana; Levis; Luteostriata; Maxima; Pseudoarundinacea; Ridleyi; Robusta; sp 'Rachel Carson'; sp. 'Bali White Stripe*; sp. 'Sumatra 375 Γ; sp. 'Widjaja 3827*; and Wrayii
[00269] Guadua: Amplexifolia; Angustifolia; angustifolia 'Bicolor'; angustifolia 'Less Thorny'; Chacoensis; Longifolia; Paniculata; sp. ureocaulis'; and Velutina
[00270] Hibanobambusa: Tranquillans; and tranquillans 'Shiroshima'
[00271] Himalayacalamus: Falconeri; falconeri 'Damarapa'; Hookerianus; Planatus; and Porcatus
[00272] Indocalamus: Cordatus; Latifolius; latifolius 'Hopei'; Longiauritus; sp. 'Hamadae'; sp. 'Solidus'; Tessellatus; and Victorialis
[00273] Indosasa: Crassiflora; and Gigantea
[00274] Lithachne: Humilis
[00275] Melocalamus: Arrectus
[00276] Melocanna: Baccifera
[00277] Menstruocalamus: Sichuanensis
[00278] Nastus: Elatus
[00279] Neohouzeaua: Mekongensis
[00280] Neololeba: Atra
[00281] Ochlandra: Stridula
[00282] Oligostachyum: Glabrescens
[00283] Olmeca: Recta
[00284] Otatea: acuminata 'Michoacan'; acuminata ssp. Acuminata; acuminata ssp. Aztecorum; acuminata ssp. aztecorum 'Dwarf; Fimbriata; and glauca 'Mayan Silver'
[00285] Oxytenanthera: Abyssinica; and Braunii
[00286] Phyllostachys: Acuta; Angusta; Arcana; arcana 'Luteosulcata'; Atrovaginata; Aurea; aurea Albovariegata'; aurea 'Dr Don'; aurea 'Flavescens-inversa'; aurea 'Holochrysa'; aurea
'Koi'; aurea 'Takemurai'; Aureosulcata; aureosulcata Alata'; aureosulcata Aureocaulis'; aureosulcata 'Harbin Inversa'; aureosulcata 'Harbin'; aureosulcata 'Pekinensis'; aureosulcata 'Spectabilis'; Aurita; Bambusoides; bambusoides Albovariegata'; bambusoides 'Castillon
Inversa'; bambusoides 'Castillon'; bambusoides 'Golden Dwarf; bambusoides 'Job's Spot'; bambusoides 'Kawadana'; bambusoides 'Marliac'; bambusoides 'Rib Leaf; bambusoides
'Richard Haubrich'; bambusoides 'Slender Crookstem'; bambusoides 'Subvariegata'; bambusoides 'Tanakae'; bambusoides 'White Crookstem'; Bissetii; bissetii 'Dwarf; Dulcis; Edulis; edulis Anderson'; edulis 'Bicolor'; edulis 'Goldstripe'; edulis 'Heterocycla'; Elegans;
Flexuosa; flexuosa 'Kimmei'; Glauca; glauca 'Notso'; glauca 'Yunzhu'; Heteroclada; heteroclada 'Purpurata'; heteroclada 'Solidstem'; Hispida; Humilis; Incarnata; Iridescens;
Kwangsiensis; Lithophila; Lofushanensis; Makinoi; mannii 'Decora'; mannii 'Mannii';
Meyeri; Nidularia; nidularia 'Farcta'; nidularia 'Smoothsheath'; Nigra; nigra 'Bory'; nigra 'Daikokuchiku'; nigra 'Hale'; nigra 'Henon'; nigra 'Megurochiku'; nigra 'Mejiro'; nigra
'Muchisasa'; nigra 'Othello'; nigra 'Punctata'; nigra 'Shimadake'; nigra 'Tosaensis'; Nuda; nuda
'Localis'; Parvifolia; Platyglossa; Praecox; praecox 'Prevernalis'; praecox 'Viridisulcata';
Prominens; Propinqua; propinqua 'Beijing'; Robustiramea; Rubromarginata; Stimulosa;
Varioauriculata; Violascens; Viridiglaucescens; Viridis; viridis 'Houzeau'; viridis 'Robert Young'; Vivax; vivax Aureocaulis'; vivax 'Black Spot'; vivax 'Huangwenzhu Inversa'; and vivax 'Huangwenzhu'
[00287] Pleioblastus: Akebono; Amarus; Argenteostriatus; Chino; chino Angustifolia'; chino 'Elegantissimus'; chino 'Kimmei'; chino 'Murakamiansus'; chino 'Vaginatus Variegatus';
Distichus; distichus 'Mini'; Fortunei; Gauntlettii; Gramineus; gramineus 'Monstrispiralis'; Hindsii; Humilis; humilis Albovariegatus'; humilis 'Variegatus'; Juxianensis; Kodzumae;
Kongosanensis; kongosanensis Akibensis'; kongosanensis Aureostriatus'; Linearis; linearis
'Nana'; Nagashima; Oleosus; Pygmaeus; pygmaeus 'Greenstripe'; pygmaeus 'Ramosissimus'; shibuyanus 'Tsuboi'; Simonii; simonii 'Variegatus'; Viridistriatus; viridistriatus
'Chrysophyllus'; and Xestrophyllus
[00288] Pseudosasa: Amabilis; Cantori; Guanxianensis; Japonica; japonica Akebono'; japonica Akebono-suji'; japonica 'Pleioblastoides'; japonica 'Tsutsumiana'; japonica
'Variegata'; Longiligula; Owatarii; Usawai; and Viridula
[00289] Raddia: Brasiliensis; and Distichophylla
[00290] Rhipidocladum: Pittieri; and Racemiflorum
[00291] Sarocalamus: Faberi; and Fangianus
[00292] Sasa: Cernua; Gracillima; Hayatae; Kagamiana; kagamiana ssp. Yoshinoi; Kurilensis; kurilensis 'Shimofuri'; Megalophylla; Nagimontana; nipponica (hort.); Oshidensis; Palmata; Senanensis; Shimidzuana; sp. Tsuboiana; and Veitchii
[00293] Sasaella: Bitchuensis; hidaensis 'muraii'; Masamuneana; masamuneana 'Albostriata'; masamuneana Aureostriata'; Ramosa; Sasakiana; and Shiobarensis
[00294] Sasamorpha: Borealis
[00295] Schizostachyum: Brachycladum; brachycladum 'Bali Kuning'; Caudatum; Glaucifolium; Jaculans; Lima; and sp. 'Murray Island'
[00296] Semiarundinaria: Fastuosa; fastuosa 'Viridis'; Fortis; Kagamiana; Lubrica; Makinoi; Okuboi; sp. Maruyamana; sp. 'Korea'; Yashadake; yashadake 'Kimmei'; and yashadake 'kimmei inversa'
[00297] Shibataea: Chinensis; Kumasaca; kumasaca Albostriata'; kumasaca Aureostriata'; Lancifolia; and Nanpingensis
[00298] Sinobambusa: Gigantea; Intermedia; Tootsik; and tootsik Albostriata'
[00299] Thamnocalamus: aristatus Aristatus hort. US'; Crassinodus; crassinodus 'Kew Beauty'; crassinodus 'Mendocino'; crassinodus 'Merlyn'; nepalensis 'Nyalam'; Spathiflorus; and Tessellatus
[00300] Thyrsostachys: Oliveri; and Siamensis
[00301] Yushania: Alpina; Anceps; anceps 'Pitt White'; Boliana; Brevipaniculata; Exilis; Maculata; and maling
[00302] Particularly useful species include: edulis; scandens; ArundinariaGigantea;
ArundinariaTecta; BambusaBalcooa; BambusaBambos; Bambusa Oldhamii;
BambusaTextilis; BambusaTulda; BashaniaFargesii; BrachystachyumDensiflorum; ChusqueaGigantea; DendrocalamusAsper; DendrocalamusBrandisii;
DendrocalamusGiganteus; DendrocalamusHamiltonii; DendrocalamusStrictus;
FargesiaDenudata; Fargesiadracocephala'Rufa'; FargesiaMurieliae; FargesiaNitida;
FargesiaRobusta; Fargesiarobusta 'Wolong'; Fargesiasp. 'Scabrida'; GuaduaAmplexifolia;
Guadua Paniculata; Himalayacalamus Falconeri; Indocalamus Tessellatus; Ochlandra Stridula; Otatea acuminata ssp. Aztecorum; Phyllostachys Atrovaginata; Phyllostachys
Aurea; Phyllostachys Bambusoides; Phyllostachys Bissetii; Phyllostachys Edulis;
Phyllostachys edulis 'Heterocycla'; Phyllostachys Glauca; Phyllostachys Iridescens;
Phyllostachys Kwangsiensis; Phyllostachys Nidularia; Phyllostachys Nigra; Phyllostachys nigra 'Henon'; Phyllostachys Nuda; Phyllostachys Parvifolia; Phyllostachys Praecox;
Phyllostachys Propinqua; Phyllostachys Viridis; Phyllostachys Vivax; Pleioblastus Distichus; Pleioblastus Fortunei; Pleioblastus Linearis; Pseudosasa Japonica; Sasa Kurilensis; Sasa Veitchii; Sasaella Masamuneana; Sasamorpha Borealis; Schizostachyum Brachycladum; Schizostachyum brachycladum 'Bali Kuning'; Schizostachyum Caudatum; Schizostachyum Glaucifolium; Schizostachyum Jaculans; Schizostachyum Lima; Schizostachyum sp. 'Murray Island'; Semiarundinaria Fastuosa; Semiarundinaria Yashadake; Shibataea Kumasaca; Sinobambusa Gigantea; Thamnocalamus Crassinodus; Thamnocalamus Tessellatus; Yushania Alpina; and Yushania maling.
[00303] As one of ordinary skill in the art appreciates, many species of bamboo have different common names. Accordingly, the following terminology and language comparisons are provided.
CHUSQUEA quila CHUSQUEA valdiviensis
DENDROCALAMUS affinis BAMBUSA emeiensis
DENDROCALAMUS membranaceus BAMBUSA membranacea
DREPANOSTACHYUM falcatum HIMALAYACALAMUS hookerianus
DREPANOSTACHYUM falcatum Var.
DREPANOSTACHYUM falconeri
sengteeanum'
HIMALAYACALAMUS falconeri
DREPANOSTACHYUM hookerianum
'Damarapa'
DREPANOSTACHYUM sengteeanum HIMALAYACALAMUS falconeri
FARGESIA angustissima BORINDA angustissima
FARGESIA crassinochis THAMNOCALAMUS crassinodus
Fargesia dracocephala Fargesia apircirubens
Fargesia dracocephala 'White Dragon ' Fargesia apircirubens 'White Dragon'
FARGESIA frigida BORINDA frigidorum
FARGESIA fungosa BORINDA fungosa
FARGESIA sp "A-4" FARGESIA adpressa
Fargesia sp. 'rufa' Fargesia dracocephala 'Rufa'
GELIDOCALAMUS fangianus SAROCALAMUS fangianus
GIGANTOCHLOA atroviolacea 'Timor
BAMBUSA lako
Black'
GIGANTOCHLOA luteostriata BAMBUSA luteostriata
GIGANTOCHLOA verticillata GIGANTOCHLOA pseudoarundinacea
Himalayacalamus asper Himalayacalamus planatus
HIMALAYACALAMUS falconeri DREPANOSTACHYUM falcatum Var. 'glomeratum' sengteeanum'
HIMALAYACALAMUS intermedins Yushania boliana
HIMALA YA CALAMUS planatus HIMALAYACALAMUS asper (hort.)
HIMALA YA CALAMUS planatus Neomicrocalamus microphyllus (hort.)
NEOMICROCALAMUS microphyllus HIMALAYACALAMUS planatus
NEOSINOCALAMUS affinis BAMBUSA emeiensis 'Chrysotrichus'
Otatea acuminata 'Mayan Silver' Otatea glauca 'Mayan Silver'
OTATEA aztecorum OTATEA acuminata ssp. aztecorum
PHYLLOSTACHYS cerata PHYLLOSTACHYS heteroclada
PHYLLOSTACHYS congesta PHYLLOSTACHYS atrovaginata
PHYLLOSTACHYS decora PHYLLOSTACHYS mannii 'Decora*
PHYLLOSTACHYS heterocycla PHYLLOSTACHYS edulis 'Heterocycla*
PHYLLOSTACHYS heterocycla pubescens PHYLLOSTACHYS edulis
PHYLLOSTACHYS heterocycla pubescens
PHYLLOSTACHYS edulis Anderson* 'Anderson '
PHYLLOSTACHYS heteroclada
PHYLLOSTACHYS purpurata
'Purpurata'
PHYLLOSTACHYS heteroclada
PHYLLOSTACHYS purpurata 'Solidstem'
'Solidstem'
PHYLLOSTACHYS purpurata
PHYLLOSTACHYS heteroclada 'Straightstem '
PLEIOBLASTUS kongosanensis
PLEIOBLASTUS akibensis
'Akibensis'
PLEIOBLASTUS gramineus 'Raseetsu- PLEIOBLASTUS gramineus
chiku' 'Monstrispiralis'
PLEIOBLASTUS variegatus PLEIOBLASTUS fortunei
Qiongzhuea tumidissinoda Chimonobambusa tumidissinoda
SASA asahinae SASA shimidzuana
SASA humilis PLEIOBLASTUS humilis
SASA pygmaea PLEIOBLASTUS pygmaeus
SASA tessellata INDOCALAMUS tessellatus
SASA variegata PLEIOBLASTUS fortunei
Sasa veitchii 'Minor' Sasa hayatae
SASAELLA glabra lbostriata' SASAELLA masamuneana Albostriata'
SASAELLA masamuneana rhyncantha SASAELLA masamuneana
SASAELLA rhyncantha SASAELLA masamuneana
SEMIAR UNDINARIA villosa SEMIARUNDINARIA okuboi
SINAR UNDINARIA FARGESIA
TETRAGONOCALAMUS angulatus CHIMONOBAMBUSA quadranqularis
THAMNOCALAMUS spathaceus FARGESIA murieliae
YUSHANIA aztecorum OTATEA acuminata ssp. aztecorum
Chinese & Japanese Names
Cephalostachyum
Xiang Nuo zhu
pergracile i
Zi zhu Phyllostachys nigra
English names
Technically not a bamboo but included within the meaning of bamboo herein.
[00304] By means of the media, kits, systems and methods described and disclosed herein, it is possible for one of ordinary skill in the art to achieve rolling tissue cultures of bamboo. As used herein, "rolling tissue culture" means that the multiplication process can continue substantially indefinitely by continuing to separate and multiply shoots. In one embodiment, one shoot is placed in a tube and the shoot multiplies into a number of additional shoots. After multiplication, each shoot or a subset of the shoots are separated and each placed in a subsequent tube for further multiplication. This process can continue while at various times, some or all shoots can be removed from the multiplication process and transitioned to ex vitro conditions. By continuing indefinitely, it is meant that 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 50, 55, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150 etc. day multiplication cycles can be repeated without initiating new explants for at least 1 month, for at least 3 months, for at least 6 months, for at least 9 months, for at least 12 months, for at least 15 months, for at least 18 months, for at least 21 months, for at least 24 months or for at least 36 months. Particular ranges of days in multiplication cycles include 1-10 days, 2-9 days, 3-6 days, 0.5-3 days, 4-5 days, 0.5 - 1 day, 10-120 days; 10-100 days; 10-80 days; 10- 60 days; 10-42 days; 10-40 days; 10-20 days; 14-120 days; 14-90 days; 14-70 days; 14-50; 14-42 days; 14-30 days; 14-21 days; 12-42 days; 20-60 days; 10-15 days; 14-20 days; 14-18 days etc.
[00305] These media systems and methods can be packaged and/or described in various kits. Kits can include, without limitation, one or more of the following in a package or container: (1) one or more media; and (2) one or more explants from one or more species of bamboo. In certain non-limiting embodiments, the media can be b-9-i media, b-9-ii media, b-9-iii media, b-9-iv media, b-9-v media, spiked b-9-i media, spiked b-9-ii media, spiked b-9-iii media, spiked b-9-iv media, spiked b-9-v media, reduced b-9-i media (reduced media are described below), reduced b-9-ii media, reduced b-9-iii media, reduced b-9-iv media, reduced b-9-v media, CW2-i media, CW2-ii media, CW2-iii media, CW2-iv media, CW2-v media, spiked CW2-i media, spiked CW2-ii media, spiked CW2-iii media, spiked CW2-iv media, spiked CW2-v media, reduced CW2-i media, reduced CW2-ii media, reduced CW2-iii media,
reduced CW2-iv media, reduced CW2-v media, b-10-i media, b-10-ii media, b-10-iii media, b-10-iv media, b-10-v media, spiked b-10-i media, spiked b-10-ii media, spiked b-10-iii media, spiked b-10-iv media, spiked b-10-v media, reduced b-10-i media, reduced b-10-ii media, reduced b-10-iii media, reduced b-10-iv media, reduced b-10-v media, b-l l-i media, b-l l-ii media, b-l l-iii media, b-l l-iv media, b-l l-v media, spiked b-l l-i media, spiked b- 11-ii media, spiked b-11-iii media, spiked b-11-iv media, spiked b-l l-v media, reduced b-11- i media, reduced b-11-ii media, reduced b-11-iii media, reduced b-11-iv media, reduced b-11- v media, b-12c-i media, b-12c-ii media, b-12c-iii media, b-12c-iv media, b-12c-v media, spiked b-12c-i media, spiked b-12c-ii media, spiked b-12c-iii media, spiked b-12c-iv media, spiked b-12c-v media, reduced b-12c-i media, reduced b-12c-ii media, reduced b-12c-iii media, reduced b-12c-iv media, reduced b-12c-v media, b-l-i media, b-l-ii media, b- 1-iii media, b-l-iv media, b-l-v media, spiked b-l-i media, spiked b-l-ii media, spiked b- 1-iii media, spiked b-l-iv media, spiked b-l-v media, reduced b-l-i media, reduced b-l-ii media, reduced b- 1-iii media, reduced b-l-iv media, reduced b-l-v media, b-4-i media, b-4-ii media, b-4-iii media, b-4-iv media, b-4-v media, spiked b-4-i media, spiked b-4-ii media, spiked b-4- iii media, spiked b-4-iv media, spiked b-4-v media, reduced b-4-i media, reduced b-4-ii media, reduced b-4-iii media, reduced b-4-iv media, reduced b-4-v media, b-6-i media, b-6-ii media, b-6-iii media, b-6-iv media, b-6-v media, spiked b-6-i media, spiked b-6-ii media, spiked b-6-iii media, spiked b-6-iv media, spiked b-6-v media, reduced b-6-i media, reduced b-6-ii media, reduced b-6-iii media, reduced b-6-iv media, reduced b-6-v media, CWl-i media, CWl-ii media, CWl-iii media, CWl-iv media, CWl-v media, spiked CWl-i media, spiked CWl-ii media, spiked CWl-iii media, spiked CWl-iv media, spiked CWl-v media, reduced CWl-i media, reduced CWl-ii media, reduced CWl-iii media, reduced CWl-iv media, reduced CWl-v media, CW3-i media, CW3-ii media, CW3-iii media, CW3-iv media, CW3-v media, spiked CW3-i media, spiked CW3-ii media, spiked CW3-iii media, spiked CW3-iv media, spiked CW3-v media, reduced CW3-i media, reduced CW3-ii media, reduced CW3-iii media, reduced CW3-iv media, reduced CW3-V media, CW4-i media, CW4-ii media, CW4-iii media, CW4-iv media, CW4-v media, spiked CW4-i media, spiked CW4-ii media, spiked CW4-iii media, spiked CW4-iv media, spiked CW4-v media, reduced CW4-i media, reduced CW4-ii media, reduced CW4-iii media, reduced CW4-iv media, reduced CW4-v media, CW5-i media, CW5-ii media, CW5-iii media, CW5-iv media, CW5-V media, spiked CW5-i media, spiked CW5-ii media, spiked CW5-iii media, spiked CW5-iv media, spiked CW5-V media, reduced CW5-i media, reduced CW5-ii media, reduced CW5-iii media, reduced CW5-iv media, reduced CW5-V media, CW6-i media, CW6-ii media, CW6-iii
media, CW6-iv media, CW6-V media, spiked CW6-i media, spiked CW6-ii media, spiked CW6-iii media, spiked CW6-iv media, spiked CW6-v media, reduced CW6-i media, reduced CW6-ii media, reduced CW6-iii media, reduced CW6-iv media, reduced CW6-V media, B- 9Ν2-Ϊ media, B-9N2-ii media, B-9N2-iii media, B-9N2-iv media, B-9N2-V media, spiked B- 9Ν2-Ϊ media, spiked B-9N2-ii media, spiked B-9N2-iii media, spiked B-9N2-iv media, spiked B-9N2-V media, reduced Β-9Ν2-Ϊ media, reduced B-9N2-ii media, reduced B-9N2-iii media, reduced B-9N2-iv media, reduced B-9N2-V media, B-12C CPPU-i media, B-12C CPPU-ii media, B-12C CPPU-iii media, B-12C CPPU-iv media, B-12C CPPU-v media, spiked B-12C CPPU-i media, spiked B-12C CPPU-ii media, spiked B-12C CPPU-iii media, spiked B-12C CPPU-iv media, spiked B-12C CPPU-v media, reduced B-12C CPPU-i media, reduced B-12C CPPU-ii media, reduced B-12C CPPU-iii media, reduced B-12C CPPU-iv media, reduced B-12C CPPU-v media, B-12C DPU-i media, B-12C DPU-ii media, B-12C DPU-iii media, B-12C DPU-iv media, B-12C DPU-v media, spiked B-12C DPU-i media, spiked B-12C DPU-ii media, spiked B-12C DPU-iii media, spiked B-12C DPU-iv media, spiked B-12C DPU-v media, reduced B-12C DPU-i media, reduced B-12C DPU-ii media, reduced B-12C DPU-iii media, reduced B-12C DPU-iv media, reduced B-12C DPU-v media, Br-2-i media, Br-2-ii media, Br-2-iii media, Br-2-iv media, Br-2-v media, spiked Br-2-i media, spiked Br-2-ii media, spiked Br-2-iii media, spiked Br-2-iv media, spiked Br-2-v media, reduced Br-2-i media, reduced Br-2-ii media, reduced Br-2-iii media, reduced Br-2-iv media, reduced Br-2-v media, Ech-i media, Ech-ii media, Ech-iii media, Ech-iv media, Ech-v media, spiked Ech-i media, spiked Ech-ii media, spiked Ech-iii media, spiked Ech-iv media, spiked Ech-v media, reduced Ech-i media, reduced Ech-ii media, reduced Ech-iii media, reduced Ech-iv media, reduced Ech-v media, Amel-i media, Amel-ii media, Amel-iii media, Amel-iv media, Amel-v media, spiked Amel-i media, spiked Amel-ii media, spiked Amel-iii media, spiked Amel-iv media, spiked Amel-v media, reduced Amel-i media, reduced Amel-ii media, reduced Amel-iii media, reduced Amel-iv media or reduced Amel-v media,. In another embodiment, the kits can comprise one or more containers for the tissue culturing process including without limitation, tubes, jars, boxes, jugs, cups, sterile bag technology, bioreactors, temporary immersion vessels, etc. In another embodiment the kits can comprise instructions for the tissue culturing of bamboo. In another embodiment, the kits comprise combinations of the foregoing. Components of various kits can be found in the same or different containers. Additionally, when a kit is supplied, the different components of the media can be packaged in separate containers and admixed immediately before use. Such
packaging of the components separately may permit long-term storage without losing the active components' functions. Alternatively, media can be provided pre-mixed.
[00306] The ingredients included in the kits can be supplied in containers of any sort such that the life of the different ingredients are preserved and are not adsorbed or altered by the materials of the container. For example, sealed glass ampoules may contain ingredients that have been packaged under a neutral, non-reacting gas, such as nitrogen. Ampoules may consist of any suitable material, such as glass, organic polymers, such as polycarbonate, polystyrene, etc., ceramic, metal or any other material typically employed to hold similar ingredients. Other examples of suitable containers include simple bottles that may be fabricated from similar substances as ampoules, and envelopes, that may comprise foil-lined interiors, such as aluminum or an alloy. Other containers include test tubes, vials, flasks, bottles, syringes, or the like. Containers may have a sterile access port, such as a bottle having a stopper that can be pierced. Other containers may have two compartments that are separated by a readily removable membrane that upon removal permits the ingredients to be mixed. Removable membranes may be glass, plastic, rubber, etc.
[00307] As stated, kits can be supplied with instructional materials. Instructions may be printed on paper or other substrate, and/or may be supplied as an electronic-readable media, such as a floppy disc, CD-ROM, DVD-ROM, Zip disc, videotape, audiotape, etc. Detailed instructions may not be physically associated with the kit; instead, a user may be directed to an internet web site specified by the manufacturer or distributor of the kit, or supplied as electronic mail.
[00308] One advantage of the disclosed embodiments is that the methods are more robust than those previously used producing plants that do not require special treatments required by those produced using methods of the prior art. For example, methods disclosed herein do not require the use of seeds or inflorescence to start plants; do not require selection of diseased starting plants (such as those exhibiting symptoms of witches broom or little leaf disease); do not require use of somatic embryogenesis and do not utilize pseudospikelets. For successful growth following tissue culture, the produced plants do not require watering directly on the pot but remain robust with overhead watering and do not require multiple adjustments to light intensity or humidity conditions prior to transfer to a greenhouse or other growing conditions. These improvements over prior methods provide even additional advantages related to the health of produced plants and efficiency of growth and processing.
[00309] Non-limiting embodiments encompassed by the present disclosure include (Stage 1, Stage 2, Stage 3, etc, media are defined elsewhere herein):
[00310] I. The following species: Arundinaria gigantea; Bambusa balcoa; Bambusa vulgaris;
Bambusa vulgaris 'Vitatta'; Bambusa Oldhamii; Bambusa tulda; endrocalamus brandesii;
Dendrocalamus asper; Dendrocalamus hamiltoni; Dendrocalamus giganteus; Dendrocalamus membranaceus; Dendrocalamus strictus; Gigantochloa aspera; Gigantochloa scortechini; Guadua culeata; uadua aculeata 'Nicaragua'; Guadua amplexifolia; Guadua angustifolia;
Guadua angustofolia bi-color; Guadua paniculata; Melocanna bambusoides; eohouzeaua dullooa (Teinostachyum); Ochlandra travancorica; Phyllostachys edulis 'Moso';
Phyllostachys nigra; Phyllostachys nigra 'Henon'; Schizostachyum lumampao;
[00311] II. Stage 1 media: b-12c-v media, B-12C-CPPU-V media, B-12C DPU-v, B-9N2-V media or b- 10-v media or spiked versions thereof;
[00312] III. Stage 2 media: CWl-v media; CW2-v media; CW3-V media; CW4-v media;
CW5-v media; CW6-V media, B-12C-CPPU-V media, B-12C DPU-v, B-9N2-V media or spiked and reduced/standard versions thereof for 10-120 day cycles (as modified for spiked media as described more fully below); and
[00313] IV. Stage 3 media: Br-2-v media; Ech-v media or Amel-v media or spiked and reduced/standard versions thereof (as modified for spiked media as described more fully below).
[00314] More particularly, the following embodiments can be used (Stage 1, Stage 2, Stage 3, etc, media are defined elsewhere herein):
[00315] Starting with a bamboo plant between the ages of 3 months and 3 years, a node from the cane with the lateral shoot just breaking the sheath can be used as the explant. Each nodal section can be cut into 3-5 millimeter sections with the shoot intact. The outer sheaths can be peeled off and discarded and the remaining nodal section piece put into a 10% bleach solution with a final concentration of 0.6% sodium hydrochloride. The explant in bleach solution can be placed onto a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) shaker table for 1 hour at 6-9 revolutions per minute. The explants can then be put into a 1% bleach solution with a final concentration of 0.06% sodium hydrochloride, and be placed back onto the shaker table for 30 minutes. This 1% bleach solution step can then be repeated.
[00316] Individual explants can then be placed on a Stage 1 media (15-25 mL) within a tube and the tubes can be placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-54

The initial Stage 1 media can be b-12c-iv at a pH of 5.7. The explants can then be transferred to fresh b-12c-iv media every 10-120 days (usually every 21 days), with contaminated tubes being discarded.
[00317] If a spiked version of the b-12c-iv media is utilized, the explants can be placed in the spiked media for 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 or 48 hours before transition to a "standard" media disclosed herein or to a media containing substantially reduced or no cytokinins ("reduced" media as used herein) for the remainder of the 10-120 day cycle. Additional time periods for placement in a spiked media include anywhere between 0.1 and 240 hours and can include, without limitation, 0.1- 0.5 hours, 0.3-2.5 hours, 2.5-6 hours, 1-10 hours, 5-15 hours, 10-20 hours, 15-25 hours, 20- 30 hours, 25-35 hours, 30-40 hours, 35-45 hours, 40-50 hours, 45-55 hours, 50-60 hours, 55- 65 hours, 60-70 hours, 65-75 hours, 70-80 hours, 75-85 hours, 80-90 hours, 85-95 hours, 90- 100 hours, 95-105 hours, 100-110 hours, 105-115 hours, 110-120 hours, 115-125 hours, 120- 130 hours, 125-135 hours, 130-140 hours, 135-145 hours, 140-150 hours, 145-155 hours, 150-160 hours, 155-165 hours, 160-170 hours, 165-175 hours, 170-180 hours, 175-185 hours, 180-190 hours, 185-195 hours, 190-200 hours, 195-205 hours, 200-210 hours, 205-215 hours, 210-220 hours, 215-225 hours, 220-230 hours, 225-235 hours, 230-240 hours, 235-245 hours, 240-250 hours, 3-6 hours, 7-17 hours, 12-22 hours, 17-27 hours, 22-32 hours, 27-37 hours, 32-42 hours, 37-47 hours, 42-52 hours, 47-57 hours, 52-62 hours, 57-67 hours, 62-72 hours, 67-77 hours, 72-82 hours, 77-87 hours, 82-92 hours, 87-97 hours, 92-102 hours, 97-107 hours, 102-112 hours, 107-117 hours, 112-122 hours, 117-127 hours, 122-132 hours, 127-137 hours, 132-142 hours, 137-147 hours, 142-152 hours, 147-157 hours, 152-162 hours, 157-167 hours, 162-172 hours, 167-177 hours, 172-182 hours, 177-187 hours, 162-172 hours, 167-177 hours, 182-192 hours, 187-197 hours, 192-202 hours, 197-207 hours, 202-212 hours, 207-217 hours, 212-222 hours, 217-227 hours, 222-232 hours, 227-237 hours, 232-242 hours, 237-247 hours or 242-252 hours. Placement in spiked media can also be 0.5 hours less than a cycle in standard or reduced media, 1 hour less than a cycle in standard or reduced media and all time periods in between 1 and 240 hours less than a cycle in standard or reduced media. Alternatively, in place of spending the remainder of the cycle in the standard or reduced media, explants can be placed on a spiked media for a period of time followed by culture on a standard or reduced media for the full cycle time (i.e. 10-120 days not reduced by time spent in the spiked media).
[00318] Media containing no cytokinins or substantially reduced cytokinins can be a reduced b-9 media, reduced CW2 media, reduced b-10 media, reduced b-11 media, reduced b-12c media, reduced b-1 media, reduced b-4 media, reduced b-6 media, reduced CW1 media, reduced CW3 media, reduced CW4 media, reduced CW5 media, reduced CW6 media,
reduced B-9N2 media, reduced B-12C CPPU media, reduced B-12C DPU media with all cytokinins and/or auxins removed or can have at least one cytokinin and/or auxin's amount reduced by 1%, 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 98%, 99%, 100%, 1-10%, 5-15%, 10-20%, 15-25%, 20- 30%, 25-35%, 30-40%, 35-45%, 40-50%, 45-55%, 50-60%, 55-65%, 60-70%, 65-75%, 70- 80%, 75-85%, 80-90%, 85-95%, 90-100%, 3-6%, 7-17%, 12-22%, 17-27%, 22-32%, 27- 37%, 32-42%, 37-47%, 42-52%, 47-57%, 52-62%, 57-67%, 62-72%, 67-77%, 72-82%, 77- 87%o, 82-92%) or 87-97%). Non-limiting examples of reduced media include (embodiments with no cytokinins or auxins not shown in table format):
Media b-12c(i-v):
Media CW4(i-v):
Component Reduced Reduced Reduced Reduced Reduced
CW4-i CW4-H CW4-iii CW4-iv CW4-v
NAA 0 0.01 0.025 0.075 0.001
BAP 0 0.75 0.25 0.5 0
IBA 0 0.1 0.15 0.01 0.2
Thidiazuron 0.01 0.1 0.15 0.2 0.15
Meta-topolin 1 2 3 4 5
Media CW1( v):
Component Reduced Reduced Reduced Reduced Reduced
CWl-i CWl-ii CWl-iii CWl-iv CWl-v
NAA 0.02 0.03 0.04 0.05 0.05
BAP 0.3 0.25 0.5 1 1
Meta-topolin 0.5 2.5 3.5 0 1
Media CW5(i- v):
Component Reduced Reduced Reduced Reduced Reduced
CW5-i CW5-ii CW5-iii CW5-iv CW5-v
NAA 0.05 0 0.025 0 0.05
BAP 0 1 0.5 0 0.5
IBA 0 0.2 0.1 0 1
Meta-topolin 0 0 2 5 4.5
Media CW6(i- v):
Component Reduced Reduced Reduced Reduced Reduced
CW6-i CW6-ii CW6-iii CW6-iv CW6-v
NAA 0 0 0.1 0 0.5
BAP 0 0 1 1 0.5
IBA 0 0 0.2 0 0.1
Meta-topolin 0 1 0 2.5 0
Thidiazuron 0.25 0 0 0.25 0
Media b-lO(i-v):
[00319] Alternatively, the cytokinins noted above can be replaced with weaker cytokinins at similar or higher levels. Exemplary weaker cytokinins include zeatin and kinetin.
[00320] Contaminated tubes can be identified by bacterial discoloration of the agar or by visible surface contamination. These explants can stay on the chosen b-12c-iv media for 3-4 10-120 day cycles (usually 21 day cycles) or as modified in the spiked procedure (spiked media for a period of 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 or 48 hours before transition to a standard or reduced media for the remainder of the 10-120 day cycle or for a full 10-120 day cycle). Additional time periods for placement in a spiked media include anywhere between 0.1 and 240 hours and can include, without limitation, 0.1-0.5 hours, 0.3-2.5 hours, 2.5-6 hours, 1-10 hours, 5-15 hours, 10-20 hours, 15- 25 hours, 20-30 hours, 25-35 hours, 30-40 hours, 35-45 hours, 40-50 hours, 45-55 hours, 50-
60 hours, 55-65 hours, 60-70 hours, 65-75 hours, 70-80 hours, 75-85 hours, 80-90 hours, 85- 95 hours, 90-100 hours, 95-105 hours, 100-110 hours, 105-115 hours, 110-120 hours, 115- 125 hours, 120-130 hours, 125-135 hours, 130-140 hours, 135-145 hours, 140-150 hours, 145-155 hours, 150-160 hours, 155-165 hours, 160-170 hours, 165-175 hours, 170-180 hours, 175-185 hours, 180-190 hours, 185-195 hours, 190-200 hours, 195-205 hours, 200-210 hours, 205-215 hours, 210-220 hours, 215-225 hours, 220-230 hours, 225-235 hours, 230-240 hours, 235-245 hours, 240-250 hours, 3-6 hours, 7-17 hours, 12-22 hours, 17-27 hours, 22-32 hours, 27-37 hours, 32-42 hours, 37-47 hours, 42-52 hours, 47-57 hours, 52-62 hours, 57-67 hours, 62-72 hours, 67-77 hours, 72-82 hours, 77-87 hours, 82-92 hours, 87-97 hours, 92-102 hours, 97-107 hours, 102-112 hours, 107-117 hours, 112-122 hours, 117-127 hours, 122-132 hours, 127-137 hours, 132-142 hours, 137-147 hours, 142-152 hours, 147-157 hours, 152-162 hours, 157-167 hours, 162-172 hours, 167-177 hours, 172-182 hours, 177-187 hours, 162-172 hours, 167-177 hours, 182-192 hours, 187-197 hours, 192-202 hours, 197-207 hours, 202-212 hours, 207-217 hours, 212-222 hours, 217-227 hours, 222-232 hours, 227-237 hours, 232-242 hours, 237-247 hours or 242-252 hours. Placement in spiked media can also be 0.5 hours less than a cycle in standard or reduced media, 1 hour less than a cycle in standard or reduced media and all time periods in between 1 and 240 hours less than a cycle in standard or reduced media. If an explant is cultured on a particular spiked media type (e.g. b-12c), when transferred to a standard or reduced media, the standard or reduced media can be of the same type (e.g. standard or reduced b-12c) or of a different type (e.g. standard or reduced CW1, CW2, CW6, b6, b9 etc.).
[00321] Explants can be taken off the media after the third cycle if multiplication is occurring. If multiplication is not occurring or not occurring to a significant degree, explants can be left on the media for a fourth cycle.
[00322] Live shoots can next be transferred to a Stage 2 media (if standard b-12c used in the previous step or a Stage 3 media if a basic spiked procedure was used), such as b-9, CW1, CW2, CW3, CW4, CW5, CW6, b-6, B-9N2, B-12C CPPU or B-12C DPU at a pH of 5.7. The cultures can stay on this Stage 2 media until the desired number of shoots is obtained by separation into new tubes and further expansion. Generally, the range of time includes 10-120 day cycles (usually 14-21 day cycles) between which the cultures are assigned to go through another multiplication round or transitioned to a Stage 3 or Stage 4 media, for example, b-10- iv or b-11-iv at a pH of 5.7 for further multiplication.
[00323] Alternatively, live shoots can also be placed on a spiked b-9, CW1, CW2, CW3, CW4, CW5, CW6, b-6, B-9N2, B-12C CPPU, B-12C DPU media for a period of 0.25, 0.5,
0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 or 48 hours before transition to a same or different type of standard or reduced media for the remainder of the 10-120 day cycle or for a full 10-120 day cycle. Additional time periods for placement in a spiked media include anywhere between 0.1 and 240 hours and can include, without limitation, 0.1-0.5 hours, 0.3-2.5 hours, 2.5-6 hours, 1-10 hours, 5-15 hours, 10-20 hours, 15- 25 hours, 20-30 hours, 25-35 hours, 30-40 hours, 35-45 hours, 40-50 hours, 45-55 hours, 50- 60 hours, 55-65 hours, 60-70 hours, 65-75 hours, 70-80 hours, 75-85 hours, 80-90 hours, 85- 95 hours, 90-100 hours, 95-105 hours, 100-110 hours, 105-115 hours, 110-120 hours, 115- 125 hours, 120-130 hours, 125-135 hours, 130-140 hours, 135-145 hours, 140-150 hours, 145-155 hours, 150-160 hours, 155-165 hours, 160-170 hours, 165-175 hours, 170-180 hours, 175-185 hours, 180-190 hours, 185-195 hours, 190-200 hours, 195-205 hours, 200-210 hours, 205-215 hours, 210-220 hours, 215-225 hours, 220-230 hours, 225-235 hours, 230-240 hours, 235-245 hours, 240-250 hours, 3-6 hours, 7-17 hours, 12-22 hours, 17-27 hours, 22-32 hours, 27-37 hours, 32-42 hours, 37-47 hours, 42-52 hours, 47-57 hours, 52-62 hours, 57-67 hours, 62-72 hours, 67-77 hours, 72-82 hours, 77-87 hours, 82-92 hours, 87-97 hours, 92-102 hours, 97-107 hours, 102-112 hours, 107-117 hours, 112-122 hours, 117-127 hours, 122-132 hours, 127-137 hours, 132-142 hours, 137-147 hours, 142-152 hours, 147-157 hours, 152-162 hours, 157-167 hours, 162-172 hours, 167-177 hours, 172-182 hours, 177-187 hours, 162-172 hours, 167-177 hours, 182-192 hours, 187-197 hours, 192-202 hours, 197-207 hours, 202-212 hours, 207-217 hours, 212-222 hours, 217-227 hours, 222-232 hours, 227-237 hours, 232-242 hours, 237-247 hours or 242-252 hours. Placement in spiked media can also be 0.5 hours less than a cycle in standard or reduced media, 1 hour less than a cycle in standard or reduced media and all time periods in between 1 and 240 hours less than a cycle in standard or reduced media.
[00324] Generally, one-ten shoots per tube can be obtained per multiplication cycle.
[00325] Following removal from the multiplication process, the shoots can be transferred to small tissue culturing boxes (known as "magenta boxes") for 10-120 days (usually 14-21 days) containing a Stage 3, Stage 4 or Stage 5 media, in this Example, BR-2 at a pH of 5.7 for 10-120 days (usually 14-21 days) or Amel at a pH of 5.7 for 10-120 days (usually 14-21 days). As above, shoots can be placed in spiked media for shorter time periods followed by placement into a standard or reduced media for the remainder of or for a full 10-120 day cycle.
[00326] As will be understood by one of ordinary skill in the art, when spiked media are used, the use of the spiked media increases the number of media stages within a particular
process due the following use of a standard or reduced media. If spiked media are used at only one stage, the process generally expands by 1 media stage. If spiked media are used at two stages, the process generally expands by 2 media stages. If spiked media are used at three stages, the process generally expands by 3 media stages, etc.
[00327] The following procedures may also be used (Stage 1, Stage 2, Stage 3, etc, media are defined elsewhere herein):
[00328] Starting with a bamboo plant between the ages of 3 months and 3 years, a node from the cane with the lateral shoot just breaking the sheath can be used as the explant. Each nodal section can be cut into 3-5 millimeter sections with the shoot intact. Some explants, including explants taken from canes 1 year or older can be pre-rinsed by shaking them in a jar of 70% isopropyl alcohol for 3 seconds followed by rinsing them under running tap water for 1 minute. Other explants are not pre-rinsed.
[00329] The outer sheaths can be peeled off and discarded and the remaining nodal section piece put into a 10% bleach solution. The explant in bleach solution can be placed onto a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) shaker table for 1 hour at 6-9 revolutions per minute. For some explants, including those taken from canes 1 year or older, this step can be modified by adding a few drops of Tween 20 to the 10% bleach solution and soaking the explants for 45 minutes rather than 1 hour. The explants can then be put into a 1% bleach solution, and placed back onto the shaker table for 30 minutes. This 1% bleach solution step can then be repeated.
[00330] Individual explants can then be placed on a Stage 1 media (15-25 mL) within a tube and the tubes placed into a regulated clean growth chamber at a temperature of from 65 °F- 70°F and a full spectrum light level of 36-90

The Stage 1 media can be standard b-12c-iv at a pH of 5.7 or spiked b-12c-iv media. If placed on standard b-12c-iv, the explants can be transferred to fresh b-12c-iv media every 10-120 days (usually every 21 days), with contaminated tubes being discarded. If on spiked b-12c-iv media, the explants can remain on the spiked media for 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 or 48 hours and then be transferred to a media without spiked components (standard or reduced) for the remainder of the 10-120 day cycle or for a full 10- 120 day cycle. Additional time periods for placement in a spiked media include anywhere between 0.1 and 240 hours and can include, without limitation, 0.1-0.5 hours, 0.3-2.5 hours, 2.5-6 hours, 1-10 hours, 5-15 hours, 10-20 hours, 15-25 hours, 20-30 hours, 25-35 hours, 30- 40 hours, 35-45 hours, 40-50 hours, 45-55 hours, 50-60 hours, 55-65 hours, 60-70 hours, 65-
75 hours, 70-80 hours, 75-85 hours, 80-90 hours, 85-95 hours, 90-100 hours, 95-105 hours, 100-110 hours, 105-115 hours, 110-120 hours, 115-125 hours, 120-130 hours, 125-135 hours, 130-140 hours, 135-145 hours, 140-150 hours, 145-155 hours, 150-160 hours, 155-165 hours, 160-170 hours, 165-175 hours, 170-180 hours, 175-185 hours, 180-190 hours, 185-195 hours, 190-200 hours, 195-205 hours, 200-210 hours, 205-215 hours, 210-220 hours, 215-225 hours, 220-230 hours, 225-235 hours, 230-240 hours, 235-245 hours, 240-250 hours, 3-6 hours, 7- 17 hours, 12-22 hours, 17-27 hours, 22-32 hours, 27-37 hours, 32-42 hours, 37-47 hours, 42- 52 hours, 47-57 hours, 52-62 hours, 57-67 hours, 62-72 hours, 67-77 hours, 72-82 hours, 77- 87 hours, 82-92 hours, 87-97 hours, 92-102 hours, 97-107 hours, 102-112 hours, 107-117 hours, 112-122 hours, 117-127 hours, 122-132 hours, 127-137 hours, 132-142 hours, 137-147 hours, 142-152 hours, 147-157 hours, 152-162 hours, 157-167 hours, 162-172 hours, 167-177 hours, 172-182 hours, 177-187 hours, 162-172 hours, 167-177 hours, 182-192 hours, 187-197 hours, 192-202 hours, 197-207 hours, 202-212 hours, 207-217 hours, 212-222 hours, 217-227 hours, 222-232 hours, 227-237 hours, 232-242 hours, 237-247 hours or 242-252 hours. Placement in spiked media can also be 0.5 hours less than a cycle in standard or reduced media, 1 hour less than a cycle in standard or reduced media and all time periods in between 1 and 240 hours less than a cycle in standard or reduced media. These explants can stay on b- 12c-iv media or spiked b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths can be removed. At the time of transfer to the third cycle, explants can be transitioned to a Stage 2 media or Stage 3 media (depending on whether spiked procedures are used), in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above or a spiked b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above for 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 or 48 hours followed by transition to standard or reduced b-12c-iv. Additional time periods for placement in a spiked media include anywhere between 0.1 and 240 hours and can include, without limitation, 0.1-0.5 hours, 0.3-2.5 hours, 2.5-6 hours, 1-10 hours, 5-15 hours, 10-20 hours, 15-25 hours, 20-30 hours, 25-35 hours, 30-40 hours, 35-45 hours, 40-50 hours, 45-55 hours, 50-60 hours, 55-65 hours, 60-70 hours, 65-75 hours, 70-80 hours, 75-85 hours, 80-90 hours, 85-95 hours, 90-100 hours, 95-105 hours, 100-110 hours, 105-115 hours, 110-120 hours, 115-125 hours, 120-130 hours, 125-135 hours, 130-140 hours, 135-145 hours, 140-150 hours, 145-155 hours, 150-160 hours, 155-165 hours, 160-170 hours, 165-175 hours, 170-180 hours, 175-185 hours, 180-190 hours, 185-195 hours, 190-200 hours, 195-205 hours, 200-210 hours, 205-215 hours, 210-220
hours, 215-225 hours, 220-230 hours, 225-235 hours, 230-240 hours, 235-245 hours, 240-250 hours, 3-6 hours, 7-17 hours, 12-22 hours, 17-27 hours, 22-32 hours, 27-37 hours, 32-42 hours, 37-47 hours, 42-52 hours, 47-57 hours, 52-62 hours, 57-67 hours, 62-72 hours, 67-77 hours, 72-82 hours, 77-87 hours, 82-92 hours, 87-97 hours, 92-102 hours, 97-107 hours, 102- 112 hours, 107-117 hours, 112-122 hours, 117-127 hours, 122-132 hours, 127-137 hours, 132-142 hours, 137-147 hours, 142-152 hours, 147-157 hours, 152-162 hours, 157-167 hours, 162-172 hours, 167-177 hours, 172-182 hours, 177-187 hours, 162-172 hours, 167-177 hours, 182-192 hours, 187-197 hours, 192-202 hours, 197-207 hours, 202-212 hours, 207-217 hours, 212-222 hours, 217-227 hours, 222-232 hours, 227-237 hours, 232-242 hours, 237-247 hours or 242-252 hours. Placement in spiked media can also be 0.5 hours less than a cycle in standard or reduced media, 1 hour less than a cycle in standard or reduced media and all time periods in between 1 and 240 hours less than a cycle in standard or reduced media. Following the third cycle, explants can be cleaned. The explants can be kept on b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above for 10-120 day cycles (usually 21 day cycles) until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months. When multiple cycles are used, explants can be cultured in standard media for all cycles, on spiked media followed by standard media for all cycles or on spiked media followed by reduced media for all cycles. Alternatively, explants can be exposed to one or more of these treatments across cycles in any combination and order.
[00331] Once an explant exhibits multiple shoots, it can be either maintained on its current media when shooting occurred (with transfer to fresh media every 10-120 days) or transferred to a subsequent media. Non-limiting subsequent media include, without limitation a b-9 media, a CW1 media, a CW2 media a CW3 media, a CW4 media, a CW5 media, a CW6 media or a b-6 media at a pH of 5.7 or spiked versions of the same followed by transition to a standard or reduced media. The cultures can stay on the current or subsequent media until the desired number of shoots is obtained by separation into new tubes and further expansion. Generally, the range of time includes 10-120 day cycles (usually 21 day cycles) between which the cultures can be assigned to go through another multiplication round or transitioned to a next stage media, such as a BR-2 media at a pH of 5.7 for 10-120 days (usually 21 days) in "magenta boxes" or a Amel media at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00332] In even more particular non-limiting embodiments, the following species can be micropropagated in the following media (at a pH of 5.5-5.7) according to procedures described in the proceeding paragraphs [000198]-[0002-l]:
[00333] Arundinaria gigantea: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00334] Bambusa balcoa: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00335] Bambusa vulgaris: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B- 12C CPPU-v, B-12C DPU-v spiked and reduced versions thereof;
[00336] Bambusa vulgaris 'Vitatta' : b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B- 9N2-v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00337] Bambusa Oldhamii: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B- 12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00338] Bambusa tulda: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00339] Dendrocalamus brandesii:b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2- v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00340] Dendrocalamus asper: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00341] Dendrocalamus hamiltoni: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2- v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00342] Dendrocalamus giganteus: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2- v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00343] Dendrocalamus membranaceus: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00344] Dendrocalamus strictus: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00345] Gigantochloa aspera: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B- 12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00346] Gigantochloa scortechini: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2- v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00347] Guadua culeata: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00348] Guadua aculeata 'Nicaragua' : b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B- 9N2-v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00349] Guadua amplexifolia: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00350] Guadua angustifolia: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B- 12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00351] Guadua angustofolia bi-color: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B- 9N2-v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00352] Guadua paniculata: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B- 12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00353] Melocanna bambusoides: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2- v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00354] Neohouzeaua dullooa (Teinostachyum): b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-v, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00355] Ochlandra travancorica: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00356] Phyllostachys edulis 'Moso' : b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B- 9N2-v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00357] Phyllostachys nigra: b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-9N2-V, B- 12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00358] Phyllostachys nigra 'Henon' : b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B-
9N2-v, B-12C CPPU-v, B-12C DPU-v or spiked and reduced versions thereof;
[00359] Schizostachyum lumampao:b-9-v, CWl-v, CW3-V, CW4-v, CW5-V, CW6-V, B- 9N2-v, B- 12C CPPU-v, B- 12C DPU-v or spiked and reduced versions thereof.
[00360] The present invention is further illustrated by the following examples that should not be construed as limiting. The contents of all references, patents, and published patent applications cited throughout this application, as well as the Figures, are incorporated herein by reference in their entirety for all purposes. In view of the many possible embodiments to which the principles of the disclosed invention may be applied, it should be recognized that the illustrated embodiments are only examples and should not be taken as limiting the scope of the invention.
EXAMPLES [00361] Example 1. Phyllostachys bissetti. Starting with a bamboo plant between the ages of 3 months and 3 years, a node from the cane with the lateral shoot just breaking the sheath was used as the explant. Each nodal section was cut into 3-5 millimeter sections with the shoot intact. The outer sheaths were peeled off and discarded and the remaining nodal section piece put into a 10% bleach solution with a final concentration of 0.6% sodium hydrochloride. The
explant in bleach solution was placed onto a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) shaker table for 1 hour at 6-9 revolutions per minute. The explants were then put into a 1% bleach solution with a final concentration of 0.06% sodium hydrochloride, and placed back onto the shaker table for 30 minutes. This 1% bleach solution step was then repeated.
[00362] Individual explants were then placed on a Stage 1 media (15-25 mL) within a tube and the tubes were placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90
The initial Stage 1 media in this Example was standard b-12c-iv at a pH of 5.7. The explants were transferred to fresh standard b-12c-iv media every 10-120 days (usually every 21 days), with contaminated tubes being discarded. Contaminated tubes were identified by bacterial discoloration of the agar or by visible surface contamination. These explants stayed on standard b-12c-iv media for 3-4 10-120 day cycles (usually 21 day cycles). Explants were taken off the media after the third cycle if multiplication was occurring. If multiplication was not occurring or not occurring to a significant degree, explants were left on the media for a fourth cycle.
[00363] Live shoots were next transferred to a Stage 2 media, in this Example, standard b-9- iv at a pH of 5.7. The cultures stayed on standard b-9-iv media until the desired number of shoots was obtained by separation into new tubes and further expansion. Generally, the range of time included 10-120 day cycles (usually 14-21 day cycles) between which the cultures were assigned to go through another multiplication round in Stage 2 media or transitioned to a Stage 3 media, in this Example, standard b-10-iv at a pH of 5.7 for further multiplication. One-ten shoots per tube were obtained per multiplication cycle.
[00364] Following removal from the multiplication process, the shoots were transferred to small tissue culturing boxes (known as "magenta boxes") for 10-120 days (usually 14-21 days) containing a Stage 3 or Stage 4 media, in this Example, standard BR-2-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00365] Example 2. Fargesia denudata. In the example of Fargeria denudata, the explants were chosen and disinfected as in Example 1. The explants were then transferred into jars containing a Stage 1 media, in this Example, standard b-12c-iv (liquid; 30-40 mL) as described in Example 1 but for the use of jars. Explants were taken off the media after the third cycle if multiplication was occurring. If multiplication was not occurring or not occurring to a significant degree, explants were left on the media for a fourth cycle. Contaminated tubes were discarded.
[00366] The cultures were then transferred onto a Stage 2 media, in this Example, standard b-l l-iv (liquid) in jars on a rotating shelf that provides 6-9 revolutions per minute. The cultures remained on standard b-11-iv media at a pH of 5.7 for 10-120 day cycles (usually 14 day cycles) until the desired number of shoots was obtained by separation into new jars and further expansion. One-fifteen shoots per jar were obtained per multiplication cycle. The shoots were then placed in a Stage 3 media, in this Example, standard Ech-iv at a pH of 6 for 10-120 days (usually 14-21 days).
[00367] Example 3. Pleioblastus fortunei. In the example of Pleioblastus fortunei, the explants were chosen and disinfected as in Example 1. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 1. Shoots were then transferred to a Stage 2 media, in this Example, standard b-9-iv in magenta boxes (40-50 mL). They remained on standard b-9-iv media for 10-120 day cycles (usually 14 day cycles) until the desired number of shoots was obtained by separation into new boxes and further expansion. One-twenty shoots per box were obtained per multiplication cycle. The shoots were then placed on a Stage 3 media, in this Example, standard BR-2-iv for 10-120 days (usually 14-21 days).
[00368] Example 4. Sasa Veitchii. In the example of Sasa Veitchii, the explants were chosen and disinfected as in Example 1. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 1. Shoots were then transferred into a Stage 2 media, in this Example, standard b-l-iv at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion One-ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 media, in this Example, standard Br-2-iv at a pH of 5.7 for 14-21 days.
[00369] Example 5. Pleioblastus viridistriatus and Thamnocalamus crassinodus. In the example of Pleioblastus viridistriatus and Thamnocalamus crassinodus, the explants were chosen and disinfected as in Example 1. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 1. Shoots were then transferred into a Stage 2 media, in this Example, standard b-4-iv at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion. One-ten shoots per tube were
obtained per multiplication cycle. The shoots were then placed in a Stage 3 media, in this Example, standard Br-2-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00370] Example 6. Chusquea Culeo "Cana Prieta." In the example of Chusquea Culeo "Cana Prieta", the explants were chosen and disinfected as in Example 1. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv media also as described in Example 1. Shoots were then transferred into a Stage 2 media, in this Example, standard b-9-iv at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion. One-ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00371] Example 7. Bambusa Old Hamii. In the example of Bambusa Old amii, the explants were chosen and disinfected as in Example 1. The explants were then transferred into boxes containing a Stage 1 media, in this Example, standard b-10-iv (40-50 mL) also as described in Example 1 but for the change to boxes. Shoots were maintained on b-10-iv media for 10- 120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new boxes and further expansion. One-twenty shoots per box were obtained per multiplication cycle. The shoots were then placed in a Stage 2 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00372] Example 8. Phyllostachys Edulis "Moso", Phyllostachys Atrovaginata & Dendrocalamus Asper. In the example of Phyllostachys Edulis "Moso", Phyllostachys Atrovaginata & Dendrocalamus Asper, the explants were chosen and disinfected as in Example 1. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 1. Shoots were then transferred into a Stage 2 media, in this Example, standard b-9-iv at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion. A standard b-6 media at a pH of 5.5 can also be used. One- ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14- 21 days).
[00373] Example 9. Guadua Angustifolia. In the example of Guadua Angustofolia, the explants were chosen and disinfected as in Example 1. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 1. Shoots were then transferred into a Stage 2 media, in this Example, standard b- 10-iv at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion. One-ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days). [00374] Example 10. Phyllostachys bissetti. Starting with a bamboo plant between the ages of 3 months and 3 years, a node from the cane with the lateral shoot just breaking the sheath was used as the explant. Each nodal section was cut into 3-5 millimeter sections with the shoot intact. Some explants, including explants taken from canes 1 year or older were pre- rinsed by shaking them in a jar of 70% isopropyl alcohol for 3 seconds followed by rinsing them under running tap water for 1 minute. Other explants were not pre-rinsed.
[00375] The outer sheaths were peeled off and discarded and the remaining nodal section piece put into a 10% bleach solution. The explant in bleach solution was placed onto a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) shaker table for 1 hour at 6-9 revolutions per minute. For some implants, including those taken from canes 1 year or older, this step was modified by adding a few drops of Tween 20 to the 10%> bleach solution and soaking the explants for 45 minutes rather than 1 hour. The explants were then put into a 1% bleach solution, and placed back onto the shaker table for 30 minutes. This 1%) bleach solution step was then repeated.
[00376] Individual explants were then placed on a Stage 1 media (15-25 mL) within a tube and the tubes were placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90

In this Example, the Stage 1 media was standard b-12c-iv at a pH of 5.7. The explants were transferred to fresh standard b-12c-iv media every 10-120 days (usually every 21 days), with contaminated tubes being discarded. These explants stayed on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv
supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months.
[00377] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example, when used standard b-9-iv at a pH of 5.7. Alternatively to using one of the b-9 media, a standard CW1 media at a pH of 5.7 can also be used. The cultures stayed on Stage 2 or Stage 3 media until the desired number of shoots was obtained by separation into new tubes and further expansion. Generally, the range of time included 10-120 day cycles (usually 21 day cycles) between which the cultures were assigned to go through another multiplication round or were transitioned to a Stage 3 or Stage 4 media, in this Example, standard BR-2-iv at a pH of 5.7 for 10-120 days (usually 21 days) in "magenta boxes".
[00378] Example 11. Fargesia denudata. In the example of Fargeria denudata, the explants were chosen and disinfected as in Example 10. The explants were then transferred into jars containing a Stage 1 media, in this Example, standard b-12c-iv (liquid; 30-40 mL) as described in Example 10 but for the use of jars. These explants stayed on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months.
[00379] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example, standard b-11-iv (liquid) at a pH of 5.7 in jars on a rotating shelf that provides 6-9 revolutions per minute. The cultures remained on Stage 2 or Stage 3 media for 10-120 day cycles (usually 14 day cycles) until the desired number of shoots was obtained by separation into new jars and further expansion. One-fifteen shoots per jar were obtained per multiplication cycle. The shoots were then placed in a Stage 3 or Stage 4 media, in this Example, standard Ech-iv at a pH of 6 for 10-120 days (usually 21 days).
[00380] Example 12. Pleioblastus fortunei. In the example of Pleioblastus fortunei, the explants were chosen and disinfected as in Example 10. The explants were then transferred
into tubes containing a Stage 1 media in this Example, standard b-12c-iv also as described in Example 10. These explants stayed on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months.
[00381] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example standard b-9-iv in magenta boxes (40-50 mL). (standard CWl media can also be used). They remained on standard b-9-iv media for 10-120 day cycles (usually 14 day cycles) until the desired number of shoots was obtained by separation into new boxes and further expansion. One-twenty shoots per box were obtained per multiplication cycle. The shoots were then placed in a Stage 3 or Stage 4 media, in this Example, standard BR-2-iv for 10-120 days (usually 14-21 days).
[00382] Example 13. Sasa Veitchii. In the example of Sasa Veitchii, the explants were chosen and disinfected as in Example 10. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 10. These explants stayed on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months.
[00383] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example standard b-l-iv at a pH of 5.5 for 10- 120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion One-ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 or Stage 4 media, in this Example, standard Br-2-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00384] Example 14. Pleioblastus viridistriatus and Thamnocalamus crassinodus. In the example of Pleioblastus viridistriatus and Thamnocalamus crassinodus, the explants were chosen and disinfected as in Example 10. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 10. These explants stayed on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months.
[00385] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example standard b-4-iv at a pH of 5.5 for 10- 120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion. One-ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 or Stage 4 media, in this Example, standard Br-2-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00386] Example 15. Chusquea Culeo "Cana Prieta". In the example of Chusquea Culeo "Cana Prieta", the explants were chosen and disinfected as in Example 10. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 10. These explants stayed on standard b-12c-iv media for 2 10- 120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months.
[00387] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example standard b-9-iv at a pH of 5.5 for 10- 120 day cycles (usually 21 days) until the desired number of shoots was obtained by separation into new tubes and further expansion. A standard b-6 media at a pH of 5.5 can also be used. One-ten shoots per tube were obtained per multiplication cycle. The shoots were
then placed in a Stage 3 or Stage 4 media, in this Example, standard Amel-iv media at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00388] Example 16. Bambusa Old Hamii. In the example of Bambusa Old amii, the explants were chosen and disinfected as in Example 10. The explants were then transferred into boxes containing a Stage 1 media, in this Example, standard b-10-iv (40-50 mL) also as described in Example 10 but for the change to boxes. These explants stayed on standard b-10- iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-10-c supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-10-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months. Cultures were maintained on Stage 2 media until the desired number of shoots was obtained. One -twenty shoots per box were obtained per multiplication cycle. The shoots were then placed in a Stage 3 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00389] Example 17. Phyllostachys Moso, Phyllostachys Atrovaginata & Dendrocalamus Asper. In the example of Phyllostachys Moso, Phyllostachys Atrovaginata and Dendrocalamus Asper, the explants were chosen and disinfected as in Example 10. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 10. These explants stayed on standard b-12c- iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months .
[00390] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example standard b-9-iv at a pH of 5.5 for 10- 120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion. A standard b-6 media at a pH of 5.5 can also
be used. One-ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 or Stage 4 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days). [00391] Example 18. Guadua Angustifolia. In the example of Guadua Angustofolia, the explants were chosen and disinfected as in Example 10. The explants were then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 10. These explants stayed on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths were removed. At the time of transfer to the third cycle, explants were transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants were cleaned. The explants were kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots were observed. Observation of multiple shoots occurred within 3-15 months.
[00392] Once the explant exhibited multiple shoots, it was either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example standard b-10-iv at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots was obtained by separation into new tubes and further expansion. One-ten shoots per tube were obtained per multiplication cycle. The shoots were then placed in a Stage 3 or Stage 4 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00393] Example 19. Phyllostachys Edulis "Moso". Starting with a bamboo plant between the ages of 3 months and 3 years, a node from the cane with the lateral shoot just breaking the sheath is used as the explant. Each nodal section is cut into 3-5 millimeter sections with the shoot intact. Some explants, including explants taken from canes 1 year or older are pre- rinsed by shaking them in a jar of 70% isopropyl alcohol for 3 seconds followed by rinsing them under running tap water for 1 minute. Other explants are not pre-rinsed.
[00394] The outer sheaths are peeled off and discarded and the remaining nodal section piece put into a 10% bleach solution. The explant in bleach solution is placed onto a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) shaker table for 1 hour at 6-9 revolutions per minute. For some implants, including those taken from canes 1 year or older, this step is modified by adding a few drops of Tween 20 to the 10%> bleach solution and soaking the explants for 45 minutes rather than 1 hour. The explants are
then put into a 1% bleach solution, and placed back onto the shaker table for 30 minutes. This 1% bleach solution step is then repeated and can then be rinsed with sterile distilled water.
[00395] Individual explants are then placed on a Stage 1 media (15-25 mL) within a tube and the tubes are placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90 μι οΐ6/ι 2/82. In this Example the Stage 1 media is spiked b-12c-iv otherwise as described in Example 10. These explants stay on spiked b-12c-iv media for 2 8-118 day cycles (usually 12 day cycles). Between cycles, excess sheaths are removed. At the time of transfer to the third cycle, explants are transitioned to a Stage 2 media, in this Example, spiked b-12c-iv supplemented with 0.5 - 3 g/L (optimum is 2 g/L) casein hydroxylate. Following the third cycle, explants are cleaned. The explants are kept on spiked b-12c-iv supplemented with 0.5 - 3 g/L casein hydroxylate for 8-118 day cycles (usually 12 day cycles) until multiple shoots were observed. Observation of multiple shoots generally occurs within 3-15 months.
[00396] Once the explant exhibits multiple shoots, it is transferred to a Stage 3 media, in this Example reduced B-9N2-iv at a pH of 5.7 for 10-120 day cycles (usually 21 day cycles). Shoots are rotated between this Stage 3 media and a Stage 4 media, in the Example, reduced B-9N2-iv liquid (agar removed) for continued rotating 10-120 day cycles (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube are generally obtained per multiplication cycle. The shoots are then placed in a Stage 5 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00397] Example 20. Phyllostachys Edulis "Moso". Explants are chosen and disinfected as in Example 19. Individual explants are then placed on a Stage 1 media (15-25 mL) within a tube and the tubes are placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90

In this Example the Stage 1 media is spiked b-12c-iii otherwise as described in Example 10. These explants stay on spiked b-12c-iii media for 2 10-120 day cycles (usually 14 day cycles) (note in this example even though the media is spiked, the time period is not shortened because b-12c-iii can be considered a weakly spiked media). Between cycles, excess sheaths are removed. At the time of transfer to the third cycle, explants are transitioned to a Stage 2 media, in this Example, reduced B-9N2-iii. Following 1 or 2 10-120 day cycles (usually 14 day cycles), explants are cleaned and placed back in Stage 1 media for additional 1 or 2 10-120 day cycles (usually 14
day cycles). The explants are kept on this rotation of Stage 1 and State 2 media until multiple shoots are observed. Observation of multiple shoots generally occurs within 3-15 months.
[00398] Once the explant exhibits multiple shoots, it is transferred to a Stage 3 media, in this Example reduced B-9N2-iii at a pH of 5.7 for 10-120 day cycles (usually 21 day cycles). Shoots are rotated between this Stage 3 media and a Stage 4 media, in the Example, reduced B-9N2-iii liquid (agar removed) for continued rotating 10-120 day cycles (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube are generally obtained per multiplication cycle. The shoots are then placed in a Stage 5 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00399] Example 21. Bambusa Old Hamii. Explants are chosen and disinfected as in Example 19. Individual explants are then placed on a Stage 1 media (15-25 mL) within a tube and the tubes are placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90
In this Example the Stage 1 media is spiked b-12c-ii otherwise as described in Example 10. These explants stay on spiked b-12c-ii media for 2 8-118 day cycles (usually 12 day cycles). Between cycles, excess sheaths are removed. Explants remain on cycles of spiked b-12c-ii media until multiple shoots were observed. Observation of multiple shoots generally occurs within 3-15 months.
[00400] Once the explant exhibits multiple shoots, it is transferred to a Stage 2 media, in this Example reduced b-10-iv at a pH of 5.7 for 10-120 day cycles (usually 21 day cycles). Shoots remain on reduced b-10-iv media for continued rotating 10-120 day cycles (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube are generally obtained per multiplication cycle. The shoots are then placed in a Stage 3 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00401] Example 22. Phyllostachys Edulis "Moso". Explants are chosen and disinfected as in Example 19. Individual explants are then placed on a Stage 1 media (15-25 mL) within a tube and the tubes are placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 200-500
In this Example the Stage 1 media is spiked b-12c-iv otherwise as described in Example 10. These explants stay on spiked b-12c-iv media for 2 8-118 day cycles (usually 12 day cycles). Between cycles, excess
sheaths are removed. Explants remain on cycles of spiked b-12c-ii media until multiple shoots were observed. Observation of multiple shoots generally occurs within 3-15 months.
[00402] Once the explant exhibits multiple shoots, it is transferred to a Stage 2 media, in this Example reduced B-9N2-iv at a pH of 5.7 for 10-120 day cycles (usually 21 day cycles). Shoots are rotated between this Stage 2 media and a Stage 3 media, in the Example, spiked b- 11 -iv for continued rotating 10-120 day cycles (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube are generally obtained per multiplication cycle. The shoots are then placed in a Stage 4 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00403] Example 23. Starting with a bamboo plant between the ages of 3 months and 3 years, a node from the cane with the lateral shoot just breaking the sheath is used as the explant. Each nodal section is cut into 3-5 millimeter sections with the shoot intact. The outer sheaths are peeled off and discarded and the remaining nodal section piece put into a 10% bleach solution with a final concentration of 0.6% sodium hydrochloride. The explant in bleach solution is placed onto a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) shaker table for 1 hour at 6-9 revolutions per minute. The explants are then put into a 1% bleach solution with a final concentration of 0.06% sodium hydrochloride, and placed back onto the shaker table for 30 minutes. This 1% bleach solution step is then repeated.
[00404] Individual explants are then placed on a Stage 1 media (15-25 mL) within a tube and the tubes are placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90
The initial Stage 1 media in this Example is spiked b-12c-iv at a pH of 5.7. The explants remain on spiked b-12c-iv media for 1-36 hours after which they are transferred to a Stage 2 media, in this Example, reduced b-12c-iv media for the remainder of a 10-120 day (usually 21 day) cycle.
[00405] Contaminated tubes are discarded. Contaminated tubes are identified by bacterial discoloration of the agar or by visible surface contamination. These explants undergo 3-4 Stage 1/2 media rotations, each including 10-120 day cycles (usually 21 day cycles). A subset of the explants are on Stage 1 spiked b-12c-iv media for 1-36 hours followed by transfer to a Stage 2 reduced b-12c-iv media for the remainder of the cycle for each cycle (rotation between Stage 1 and Stage 2 media). Other explants alternate between culture on the spiked/reduced protocol and culture on standard b-12c-iv media (rotation between Stage 1,
Stage 2 and Stage 3 media). Alternatively, explants could begin the process in standard b- 12c-iv media for the first 10-120 day cycle and then transition to the spiked/reduced protocol for one or more of the following cycles.
[00406] Explants are taken off the media after the third cycle if multiplication is occurring. If multiplication is not occurring or not occurring to a significant degree, explants are left on media for a fourth cycle. Spiked/reduced or standard media is chosen based on the particular explant's previous treatment and treatment parameters (all cycles on spiked/reduced or alternating between spiked/reduced and standard).
[00407] Live shoots are next transferred to a Stage 3 or Stage 4 media (depending on previous treatments), in this Example, standard b-9-iv at a pH of 5.7. The cultures stay on b- 9-iv media until the desired number of shoots is obtained by separation into new tubes and further expansion. Generally, the range of time includes 10-120 day cycles (usually 14-21 day cycles) between which the cultures are assigned to go through another multiplication round in Stage 3 or Stage 4 media or transitioned to a Stage 4 or Stage 5 media, in this Example, standard b-10-iv at a pH of 5.7 for further multiplication. One-ten shoots per tube can be obtained per multiplication cycle.
[00408] Following removal from the multiplication process, the shoots are transferred to small tissue culturing boxes (known as "magenta boxes") for 10-120 days (usually 14-21 days) containing a Stage 5 or Stage 6 media, in this Example, standard BR-2-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00409] Example 24. Explants are chosen and disinfected as in Example 19. The explants are then transferred into jars containing a Stage 1 media, in this Example, standard b-12c-iv with a pH of 5.7 (liquid; 30-40 mL) in a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90
The explants are transferred to fresh standard b-12c-iv media every 10-120 days (usually every 21 days), with contaminated tubes being discarded. Contaminated tubes are identified by bacterial discoloration of the agar or by visible surface contamination. These explants stay on standard b-12c-iv media for 3-4 10-120 day cycles (usually 21 day cycles).
[00410] The cultures are then transferred onto a Stage 2 media, in this Example, spiked b- 11-iv (liquid) in jars on a rotating shelf that provides 6-9 revolutions per minute. The cultures remained on spiked b-11-iv media at a pH of 5.7 for 0.5-12 hours and are then transferred to Stage 3 reduced b-12c-iv media for the remainder of the 10-120 day cycle (usually 14 day cycles) until the desired number of shoots can be obtained by separation into new jars and
further expansion. One-fifteen shoots per jar can be obtained per multiplication cycle. The shoots are then placed in a Stage 4 media, in this Example, standard Ech-iv at a pH of 6 for 10-120 days (usually 14-21 days). [00411] Example 25. Explants are chosen and disinfected as in Example 19. The explants are then transferred into tubes containing a Stage 1 media, in this Example, spiked b-12c-iv for a period of 24-48 hours followed by transfer of the cultures to a Stage 2 reduced b-12c-iv media for 10-21 days (generally 14).
[00412] Shoots are then transferred to a Stage 3 media, in this Example, spiked b-9-iv in magenta boxes (40-50 mL). They remain on spiked b-9-iv media for 12-36 hours followed by transfer to Stage 4 reduced b-9-iv media or reduced CW-2-iv media for the remainder of the 10-120 day cycles (usually 14 day cycles) until the desired number of shoots is obtained by separation into new boxes and further expansion. One-twenty shoots per box can be obtained per multiplication cycle. The shoots are then placed on a Stage 5 media, in this Example, spiked BR-2-iv for 5-10 hours followed by transfer to Stage 6 reduced BR-2-iv for the remainder of the 10-120 day (usually 14-21 days) cycle.
[00413] Example 26. Explants are chosen and disinfected as in Example 19. The explants are then transferred into tubes containing a Stage 1 media, in this Example, spiked b-12c-ii at a pH of 5.7. Explants remain on spiked b-12c-ii media for 3-18 hours followed by transfer to standard b-12c-iv media for 10-21 days (usually 14 days). Three-eighteen hours in spiked b- 12c-ii followed by 10-21 days in standard b-12c-iv cycles are repeated until explants begin to multiply. Shoots are then transferred into a Stage 3 media, in this Example, standard b-l-iv at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 4 media, in this Example, spiked Br-2-iv at a pH of 5.7 for 1-10 hours followed by standard Br-2-iv for 14-21 days. [00414] Example 27. Explants are chosen and disinfected as in Example 19. The explants are then transferred into tubes containing a Stage 1 media, in this Example, spiked b-12c-iv for 1-24 hours. Shoots are then transferred into a Stage 2 media, in this Example, standard b- 10-iv media for 10-120 days (generally 14). Rotation through Stage 1 and Stage 2 media continues until explants begin to multiply. Once multiplication has begun, shoots are
transferred to standard b-4-iv media at a pH of 5.5 for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 4 media, in this Example, spiked Br-2-iv at a pH of 5.7 for 1-10 hours followed by the Stage 5 media, standard Amel-iv for 10-120 days (usually 14-21 days).
[00415] Example 28. Explants are chosen and disinfected as in Example 19. The explants are then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv media as described in Example 1. Shoots are then transferred into a Stage 2 media, in this Example, spiked b-9-iv at a pH of 5.5 for 4-24 hours. Following culture in the spiked b-9-iv media for 4-24 hours, shoots are placed in Stage 3 standard b-9-iv media for 10-120 days (usually 21 days). Rotation between spiked and standard b-9-iv media is repeated until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 4 media, in this Example, Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00416] Example 29. Explants are chosen and disinfected as in Example 19. The explants are then transferred into boxes containing a Stage 1 media, in this Example, standard b-10-iv (40-50 mL). Shoots are maintained on a rotation of standard b-10-iv media (10-120 day cycles (usually 21 day cycles)), Stage 2 spiked b-10-iv media (1-10 hours) and Stage 3 reduced b-10-iv media (10-120 day cycles (usually 10 day cycles) until the desired number of shoots is obtained by separation into new boxes and further expansion. One-twenty shoots per box can be obtained per multiplication cycle. The shoots are then placed in a Stage 4 media, in this Example, Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00417] Example 30. Explants are chosen and disinfected as in Example 19. The explants are then transferred into tubes containing a Stage 1 media, in this Example, spiked b-12c-i for 1-24 hours followed by Stage 2 reduced b-10-i for 10-21 days (generally 14) for 3 or 4 cycles. Shoots are then transferred into a Stage 3 media, in this Example, spiked b-9-iv at a pH of 5.5 for 1-5 hours followed by transfer to a stage 4, standard b-9-iv media for 10-120 day cycles (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. A spiked and standard or reduced B-6 media
rotation at a pH of 5.5 can also be used. One-ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 5 media, in this Example, Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days). [00418] Example 31. Explants are chosen and disinfected as in Example 19. The explants are then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv as described in Example 1. Shoots are then transferred into a Stage 2 media, in this Example, spiked b-10-iv at a pH of 5.5 for 0.5-3 hours followed by standard b-10-iv media as a Stage 3 media for 10-120 day cycles (usually 21 day cycles). The spiked to standard b-10-iv cycles are repeated until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 3 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days). [00419] Example 32. Starting with a bamboo plant between the ages of 3 months and 3 years, a node from the cane with the lateral shoot just breaking the sheath is used as the explant. Each nodal section is cut into 3-5 millimeter sections with the shoot intact. Some explants, including explants taken from canes 1 year or older are pre-rinsed by shaking them in a jar of 70% isopropyl alcohol for 3 seconds followed by rinsing them under running tap water for 1 minute. Other explants are not pre-rinsed.
[00420] The outer sheaths are peeled off and discarded and the remaining nodal section piece put into a 10% bleach solution. The explant in bleach solution is placed onto a Lab Rotators, Adjustable speed, Barnstead/Lab line orbital Shaker (model number KS 260) shaker table for 1 hour at 6-9 revolutions per minute. For some implants, including those taken from canes 1 year or older, this step is modified by adding a few drops of Tween 20 to the 10%> bleach solution and soaking the explants for 45 minutes rather than 1 hour. The explants are then put into a 1% bleach solution, and placed back onto the shaker table for 30 minutes. This 1%) bleach solution step is then repeated.
[00421] Individual explants are then placed on a Stage 1 media (15-25 mL) within a tube and the tubes are placed into a regulated clean growth chamber at a temperature of from 65°F-70°F and a full spectrum light level of 36-90

In this Example, the Stage 1 media is standard b-12c-iv at a pH of 5.7. The explants are transferred to fresh b-12c-iv media every 10-120 days (usually every 21 days), with contaminated tubes being discarded. These explants stay on b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles).
Between cycles, excess sheaths are removed. At the time of transfer to the third cycle, explants are transitioned to a Stage 2 media, in this Example, spiked b-12c-i supplemented with 7 g/L carageenan (rather than the 5.5 g/L provided above) for a period of 0.5-10 hours followed by placement in standard b-12c-iv for 10-120 days (usually 21). Following the third cycle, explants are cleaned. The explants are kept on a rotation of these Stage 2 and Stage 3 media for the stated time periods until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months.
[00422] Once the explant exhibits multiple shoots, it is either maintained on its Stage 2 media/Stage 3 media rotation or transferred to a Stage 4 media, in this Example, when used standard b-9-iv at a pH of 5.7. Alternatively to using one of the b-9 media, a CW1 media at a pH of 5.7 can also be used. The cultures stay on Stage 2/3 media or Stage 4 media until the desired number of shoots is obtained by separation into new tubes and further expansion. Generally, the range of time includes 10-120 day cycles (usually 21 day cycles) between which the cultures are assigned to go through another multiplication round or are transitioned to a Stage 4 or Stage 5 media, in this Example, standard BR-2-iv at a pH of 5.7 for 10-120 days (usually 21 days) in "magenta boxes".
[00423] Example 33. Explants are chosen and disinfected as in Example 28. The explants are then transferred into jars containing a Stage 1 media, in this Example, spiked b-12c-iv (liquid; 30-40 mL). These explants stay on spiked b-12c-iv media for 0.5-24 hours followed by placement in Stage 2 reduced b-12c-i for 10-120 days (usually 21). This process is repeated two times. Between cycles, excess sheaths are removed.
[00424] At the time of transfer to the third cycle, explants are transitioned to a Stage 3 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan (rather than the 5.5 g/L) for 10-120 day cycles (usually 21 day cycles). Following the third cycle, explants are cleaned. The explants are kept on the Stage 3 standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months.
[00425] Once the explant exhibits multiple shoots, it is either maintained on its Stage 3 media or transferred to a Stage 4 media, in this Example, spiked b-l l-iv (liquid) at a pH of 5.7 in jars on a rotating shelf that provides 6-9 revolutions per minute. Shoots remain on spiked b-l l-iv for 0.5-24 hours followed by transition to a Stage 5 standard b-l l-iv. The cultures remain on Stage 3 or Stage 4/5 media for 10-120 day cycles (usually 14 day cycles) until the desired number of shoots is obtained by separation into new jars and further
expansion. One-fifteen shoots per jar can be obtained per multiplication cycle. The shoots are then placed in a Stage 4 or Stage 6 media, in this Example, spiked Ech-iv at a pH of 6 for 1- 24 hours. Shoots are then transitioned to a Stage 5 or 7 media that is standard Ech-iv for 10- 120 days (usually 21 days).
[00426] Example 34. Explants are chosen and disinfected as in Example 28. The explants are then transferred into tubes containing a Stage 1 media in this Example, spiked b-12c-iv. These explants stay on spiked b-12c-iv media for 0.5-24 hours followed by transfer to a Stage 2 standard b-12c-iv media for 10-120 day cycles (usually 21 day cycles). This rotation is repeated 3 times. Between cycles, excess sheaths are removed. At the time of transfer to the fourth cycle, explants are transitioned to a Stage 3 media, in this Example, spiked b-12c-iv supplemented with 7 g/L carageenan (rather than the 5.5 g/L) for 1-24 hours followed by transfer to Stage 4 reduced b-12c-ii media. Following the fourth cycle, explants are cleaned. The explants are kept on Stage 3/4 media rotation for 10-120 day cycles (usually 21 day cycles) until multiple shoots are observed. Observation of multiple shoots can occur within 3- 15 months.
[00427] Once the explant exhibits multiple shoots, it is either maintained on its Stage 3/4 media or transferred to a Stage 5 media, in this Example standard b-9-iv in magenta boxes (40-50 mL) (CW1 media or spiked and reduced and/or standard versions thereof can also be used). They remain on b-9-iv media for 10-120 day cycles (usually 14 day cycles) until the desired number of shoots is obtained by separation into new boxes and further expansion. One-twenty shoots per box can be obtained per multiplication cycle. The shoots are then placed in a Stage 5 or Stage 6 media, in this Example, BR-2-iv for 10-120 days (usually 14- 21 days).
[00428] Example 35. Explants are chosen and disinfected as in Example 28. The explants are then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv as described in Example 28. These explants stay on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths are removed. At the time of transfer to the third cycle, explants are transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan (rather than the 5.5 g/L). Following the third cycle, explants are cleaned. The explants are kept on standard b-12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months.
[00429] Once the explant exhibits multiple shoots, it is either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example spiked b-l-iv at a pH of 5.5 for 5-10 hours followed by transfer to Stage 4 no cytokinin b-1 media for 10-120 day cycles (usually 21 day cycles). The stage 3/4 media rotation is repeated until the desired number of shoots is obtained by separation into new tubes and further expansion. One -ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 3 or Stage 5 media, in this Example, spiked Br-2-iv at a pH of 5.7 for 1-10 hours followed by reduced Br-2-i for 10- 120 days (usually 14-21 days). [00430] Example 36. Explants are chosen and disinfected as in Example 28. The explants are then transferred into tubes containing a Stage 1 media, in this Example, spiked b-12c-iv. These explants stay on spiked b-12c-iv media for 0.5-10 hours followed by transfer to a Stage 2 no cytokinin b-12c media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths are removed. At the time of transfer to the third cycle, explants are transitioned to a Stage 3 media, in this Example, spiked b-12c-iii supplemented with 7 g/L carageenan (rather than the 5.5 g/L) for 0.5-10 hours followed by transfer back to the Stage 2 no cytokinin b-12c media. Following the third cycle, explants are cleaned. The explants are kept on the Stage 3/2 media rotation for 10-120 day cycles (usually 21 day cycles) until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months.
[00431] Once the explant exhibits multiple shoots, it is either maintained on its Stage 3/2 media rotation or transferred to a Stage 4 media, in this Example spiked b-4-iv at a pH of 5.5 for 12-36 hours followed by Stage 5 no cytokinin b-4-iv for 10-120 days (usually 21 day cycles) until the desired number of shoots is obtained by separation into new tubes and further expansion. One-ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 4 or Stage 6 media, in this Example, Br-2-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00432] Example 37. Explants are chosen and disinfected as in Example 28. The explants are then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv also as described in Example 28. These explants stay on b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths are removed. At the time of transfer to the third cycle, explants are transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided above. Following the third cycle, explants are cleaned. The explants are kept on standard b-
12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months.
[00433] Once the explant exhibits multiple shoots, it is either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example standard b-9-iv at a pH of 5.5 for 10- 120 day cycles (usually 21 days) until the desired number of shoots is obtained by separation into new tubes and further expansion. A b-6 media at a pH of 5.5 can also be used. One-ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 3 or Stage 4 media, in this Example, spiked Amel-iv media at a pH of 5.7 for 0.5-24 hours followed by transfer to a Stage 4 or 5 no cytokinin Amel media for 10-120 days (usually 14-21 days).
[00434] Example 38. Explants are chosen and disinfected as in Example 28. The explants are then transferred into boxes containing a Stage 1 media, in this Example, spiked b-10-iv (40-50 mL). Explants remain on spiked b-10-iv media for 1-5 hours and are then transferred to a Stage 2 no-cytokinin b-10 media for 1-5 days followed by transfer to Stage 3 reduced b- 10-ii media for 5-115 days (usually 18 days). Between cycles, excess sheaths are removed, and the rotation is repeated two times. At the time of transfer to the third rotation, explants are transitioned to a Stage 4 media, in this Example, spiked b-lOc-i supplemented with 7 g/L carageenan (rather than the 5.5 g/L) for 1-5 hours, followed by transfer to a Stage 5 no cytokinin b-lOc for 1-5 days and Stage 6 reduced b-12c for 5-115 days (usually 18 days). Following the third cycle, explants are cleaned. The explants are kept on the Stage 4/5/6 media rotation until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months. Cultures are maintained on the Stage 4/5/6 media rotation until the desired number of shoots is obtained. One-twenty shoots per box can be obtained per multiplication cycle. The shoots are then placed in a Stage 7 media, in this Example, standard Amel-iv at a pH of 5.7 for 10-120 days (usually 14-21 days).
[00435] Example 39. Explants are chosen and disinfected as in Example 28. The explants are then transferred into tubes containing a Stage 1 media, in this Example, standard b-12c-iv as described in Example 28. These explants stay on standard b-12c-iv media for 2 10-120 day cycles (usually 21 day cycles). Between cycles, excess sheaths are removed. At the time of transfer to the third cycle, explants are transitioned to a Stage 2 media, in this Example, standard b-12c-iv supplemented with 7 g/L carageenan rather than the 5.5 g/L provided
above. Following the third cycle, explants are cleaned. The explants are kept on standard b- 12c-iv supplemented with 7 g/L carageenan for 10-120 day cycles (usually 21 day cycles) until multiple shoots are observed. Observation of multiple shoots can occur within 3-15 months.
[00436] Once the explant exhibits multiple shoots, it is either maintained on its Stage 2 media or transferred to a Stage 3 media, in this Example spiked b-9-iv at a pH of 5.5 for 0.5- 24 hours followed by transfer to a Stage 4 no cytokinin b-4 media for 10-120 days. The Stage 2 or Stage 3/4 media rotation cycles (usually 21 day cycles) continue until the desired number of shoots is obtained by separation into new tubes and further expansion. A spiked or standard b-6 media at a pH of 5.5 can also be used. One -ten shoots per tube can be obtained per multiplication cycle. The shoots are then placed in a Stage 3 or Stage 5 media, in this Example, spiked Amel-iv at a pH of 5.7 for 24 hours followed by transfer to standard Amel- iv for 10-120 days (usually 14-21 days).
[00437] As will be understood by one of ordinary skill from the provided examples, the tissue culturing method for individual species includes slight variations in media, timing and growth conditions. These variations for individual species require optimization based on factors including location, desired outcome, starting material, etc.
[00438] For each of the species provided in the examples listed above, in particular embodiments, each can be initiated and/or multiplied in b-9-i media, b-9-ii media, b-9-iii media, b-9-iv media, b-9-v media, spiked b-9-i media, spiked b-9-ii media, spiked b-9-iii media, spiked b-9-iv media, spiked b-9-v media, reduced b-9-i media (reduced media are described below), reduced b-9-ii media, reduced b-9-iii media, reduced b-9-iv media, reduced b-9-v media, CW2-i media, CW2-ii media, CW2-iii media, CW2-iv media, CW2-v media, spiked CW2-i media, spiked CW2-ii media, spiked CW2-iii media, spiked CW2-iv media, spiked CW2-v media, reduced CW2-i media, reduced CW2-ii media, reduced CW2-iii media, reduced CW2-iv media, reduced CW2-v media, b-10-i media, b-10-ii media, b-10-iii media, b-10-iv media, b-10-v media, spiked b-10-i media, spiked b-10-ii media, spiked b-10-iii media, spiked b-10-iv media, spiked b-10-v media, reduced b-10-i media, reduced b-10-ii media, reduced b-10-iii media, reduced b-10-iv media, reduced b-10-v media, b-l l-i media, b-l l-ii media, b-l l-iii media, b-l l-iv media, b-l l-v media, spiked b-l l-i media, spiked b- 11-ii media, spiked b-11-iii media, spiked b-11-iv media, spiked b-l l-v media, reduced b-11- i media, reduced b-11-ii media, reduced b-11-iii media, reduced b-11-iv media, reduced b-11- v media, b-12c-i media, b-12c-ii media, b-12c-iii media, b-12c-iv media, b-12c-v media, spiked b-12c-i media, spiked b-12c-ii media, spiked b-12c-iii media, spiked b-12c-iv media,
spiked b-12c-v media, reduced b-12c-i media, reduced b-12c-ii media, reduced b-12c-iii media, reduced b-12c-iv media, reduced b-12c-v media, b-l-i media, b-l-ii media, b-l-iii media, b-l-iv media, b-l-v media, spiked b-l-i media, spiked b-l-ii media, spiked b-l-iii media, spiked b-l-iv media, spiked b-l-v media, reduced b-l-i media, reduced b-l-ii media, reduced b-l-iii media, reduced b-l-iv media, reduced b-l-v media, b-4-i media, b-4-ii media, b-4-iii media, b-4-iv media, b-4-v media, spiked b-4-i media, spiked b-4-ii media, spiked b-4- iii media, spiked b-4-iv media, spiked b-4-v media, reduced b-4-i media, reduced b-4-ii media, reduced b-4-iii media, reduced b-4-iv media, reduced b-4-v media, b-6-i media, b-6-ii media, b-6-iii media, b-6-iv media, b-6-v media, spiked b-6-i media, spiked b-6-ii media, spiked b-6-iii media, spiked b-6-iv media, spiked b-6-v media, reduced b-6-i media, reduced b-6-ii media, reduced b-6-iii media, reduced b-6-iv media, reduced b-6-v media, CWl-i media, CWl-ii media, CWl-iii media, CWl-iv media, CWl-v media, spiked CWl-i media, spiked CWl-ii media, spiked CWl-iii media, spiked CWl-iv media, spiked CWl-v media, reduced CWl-i media, reduced CWl-ii media, reduced CWl-iii media, reduced CWl-iv media, reduced CWl-v media, CW3-i media, CW3-ii media, CW3-iii media, CW3-iv media, CW3-v media, spiked CW3-i media, spiked CW3-ii media, spiked CW3-iii media, spiked CW3-iv media, spiked CW3-v media, reduced CW3-i media, reduced CW3-ii media, reduced CW3-iii media, reduced CW3-iv media, reduced CW3-V media, CW4-i media, CW4-ii media, CW4-iii media, CW4-iv media, CW4-v media, spiked CW4-i media, spiked CW4-ii media, spiked CW4-iii media, spiked CW4-iv media, spiked CW4-v media, reduced CW4-i media, reduced CW4-ii media, reduced CW4-iii media, reduced CW4-iv media, reduced CW4-v media, CW5-i media, CW5-ii media, CW5-iii media, CW5-iv media, CW5-V media, spiked CW5-i media, spiked CW5-ii media, spiked CW5-iii media, spiked CW5-iv media, spiked CW5-V media, reduced CW5-i media, reduced CW5-ii media, reduced CW5-iii media, reduced CW5-iv media, reduced CW5-V media, CW6-i media, CW6-ii media, CW6-iii media, CW6-iv media, CW6-V media, spiked CW6-i media, spiked CW6-ii media, spiked CW6-iii media, spiked CW6-iv media, spiked CW6-v media, reduced CW6-i media, reduced CW6-ii media, reduced CW6-iii media, reduced CW6-iv media, reduced CW6-V media, B- 9Ν2-Ϊ media, B-9N2-ii media, B-9N2-iii media, B-9N2-iv media, B-9N2-V media, spiked B- 9Ν2-Ϊ media, spiked B-9N2-ii media, spiked B-9N2-iii media, spiked B-9N2-iv media, spiked B-9N2-V media, reduced Β-9Ν2-Ϊ media, reduced B-9N2-ii media, reduced B-9N2-iii media, reduced B-9N2-iv media, reduced B-9N2-V media, B-12C CPPU-i media, B-12C CPPU-ii media, B-12C CPPU-iii media, B-12C CPPU-iv media, B-12C CPPU-v media, spiked B-12C CPPU-i media, spiked B-12C CPPU-ii media, spiked B-12C CPPU-iii media,
spiked B-12C CPPU-iv media, spiked B-12C CPPU-v media, reduced B-12C CPPU-i media, reduced B-12C CPPU-ii media, reduced B-12C CPPU-iii media, reduced B-12C CPPU-iv media, reduced B-12C CPPU-v media, B-12C DPU-i media, B-12C DPU-ii media, B-12C DPU-iii media, B-12C DPU-iv media, B-12C DPU-v media, spiked B-12C DPU-i media, spiked B-12C DPU-ii media, spiked B-12C DPU-iii media, spiked B-12C DPU-iv media, spiked B-12C DPU-v media, reduced B-12C DPU-i media, reduced B-12C DPU-ii media, reduced B-12C DPU-iii media, reduced B-12C DPU-iv media, reduced B-12C DPU-v media, or combinations thereof according to the various procedures described herein.
[00439] As used herein "in" and "on" are interchangeable in the context of placing explants, shoots or plantlets within a tube, jar, box or jug containing a media.
Example 40. Pulp Produced From Pure Stand Bamboo
Materials and Methods
[00440] Mature Bamboo stalks were provided. The material was grown on a research plot. The green culms were harvested and chipped by a disc chipper. The chips were screened with accepts set at 2mm and 8mm holes and were cold stored (4°C) at an approximately 40% moisture content prior to use. Chipping resulted in material very similar to traditional chipped wood material.
[00441] NM-6 hybrid poplar, Populus maximowiczii x nigra, trees were harvested after 10 years of age and seasoned for 3 months before use. The pulp was hand debarked, chipped and screened with accepts set at 2mm and 8mm holes. The chipped were allowed to air-dry to approximately 25% moisture and cold stored until used. Chemicals utilized for the pulping and bleaching reactions were purchased from VWR International and used as received.
[00442] The pulping experiments were performed at three scales including 5L reactors placed into a rotating steam jacketed reactor, a 20L circulating digester with an indirect heat exchanger, and a pilot scale 400L rotating reactor with steam jacket heating.
[00443] Bleaching was performed on a lab scale using a Quantum Reactor and on a pilot scale utilizing the rotating reactor for the oxygen delignification and a continuous recirculating 800L tower for chlorine dioxide and hydrogen peroxide-reinforced alkaline extraction stages. Samples were well-washed between bleach sequences with lab samples employing a centrifuge with a cloth filter and the pilot scale employed a rotating screen washer with vacuum dewatering. Elemental chlorine free chlorine dioxide was prepared through acidification of sodium chlorite by H2S04 and collection of the C102 gas into chilled water after passing through a NaC103 solution trap.
[00444] Physical properties were measured on handsheets after they were refined in a PFI mill prepared following TAPPI Standards T-205 and T-248. Freeness, basis weight, tensile, caliper and tear were measured utilizing TAPPI Standards T220, T411, and T414. [00445] Lab Experiments
[00446] Cooking conditions for bamboo were pre-screened by pulping approximately lOOg of bamboo chips in rotating batch reactors. Effective alkali was varied between 15 and 21% and sulfidity between 25 and 35% keeping the rest of pulping parameters constant at a liquor to wood ration of 5 to 1, H-Factor at 1100, and a maximum cooking temperature of 165°C. Results of fiber yield and kappa testing are shown below in Figure 2. Based on this data the conditions of 17% effective alkali and 35% sulfidity were selected to maximize pulping yield while achieve delignification to approximately a 20 kappa number. These results are consistent with other bamboo species studies utilizing kraft pulping and oxygen delignification6' 7.
[00447] Cooking conditions for the poplar were also pre-screened by pulping approximately 100 g of poplar chips in rotating batch reactors. Effective alkali was again varied between 15 and 21 ), with lower sulfidities between 24 and 30%>, which is more typical for wood species. As in the bamboo experiments, the rest of the pulping parameters were held constant using a liquor to wood ratio of 5 to 1, an H-Factor of 1100, and a maximum cooking temperature of 165°C. Results of fiber yield and kappa testing are shown below in Fig. 3. The data suggest a lower sensitivity to sulfidity than the bamboo chips, with 27% and 30% giving similar results. Based on this data, the conditions of 19%> effective alkali and 27% sulfidity were selected to maximize pulping yields, while achieving delignification to approximately a 20 kappa number, which is consistent with typical hardwood kraft cooking conditions.
Pulp production
[00448] Three larger batch cooks of 250 grams of chips were digested to produce pulp for subsequent lab bleaching sequence using a 20L circulating digester with indirect heat exchanger following the conditions shown in Table 1. The cook resulted in slightly higher yield and kappa numbers and improved yield as compared to the initial cooks, presumably due to better liquor/chip mixing and heat transfer in the larger circulation reactor.
Table 1. Scaled-up Pulping Conditions
Stage Moso Poplar
Effective Alkali 17% 19%
Sulfidity 35% 27%
H-Factor 1100 1100
Maximum Temperature 165°C 165°C
Liquor to Biomass ratio 5 to 1 5 to 1
Kappa # (std dev) 23.4 (0.7) 18.5 (1.4)
Screened Yield 43.3% (1.0%) 48.8% (1.2%)
Bleaching Experiments
[00449] The resulting pulps from the larger cooks were then combined and bleached using a Quantum Reactor following an OD(EP)D sequence utilizing the conditions shown in Table 2, which represents a typical sequence used for hardwood pulp8.
Table 2. Bleaching Conditions.
Stage Conditions
O Pressurized with 02 to 80 psig, 10% esc, 2.5% NaOH, 60 min.,
varying temperatures
D 0.20 Kappa factor, 3.5%> esc, 50 C, 45 min., initial pH adjusted to
between 4 and 5 with 10% H2S04
EP 0.5% H202, 10% esc, 70°C, 60 min., 2.5% NaOH, terminal pH ~ 11.0
D Varying % C102, 10%> esc, 75 C, 120 min., pH adjustment to achieve
final pH between 4.5 and 5.5 with 10% H2S04
[00450] With an incoming kappa number of 18 and 23 the pulps still contain approximately 3% lignin content. The oxygen stage is employed as a low cost method to further delignify but at conditions mild enough to not further degrade cellulose or lower the yield. Consistency, caustic charge, temperature, and oxygen in this stage were held constant, with conditions and pressure set to have excess oxygen. Temperature was varied until the kappa number was reduced by 40%> to minimize cellulose degradation and yield loss, with the
results shown in Table 3. The results suggest a similar response between the two pulps with an optimal oxygen delignification between 100 and 110°C.
Table 3. Oxygen Delignification Results.
Moso Poplar
Temperature Initial Final % delig Initial Final % delig
(°C) Kappa Kappa Kappa Kappa
110 23.4 11.2 52% 18.5 9.9 46%
100 23.4 15.4 34% 18.5 10.4 44%
90 23.4 16.1 31% 18.5 12.4 33% [00451] The 100°C oxygen delignification step was repeated and utilized for the next two bleaching stages, D and E(P), which were designed to finish the delignification and remove the majority of the chromophores. Conditions shown in Table 1, were not varied for these stages, which were modeled after bleaching hardwood pulp. The final chlorine dioxide stage was done with varying charges to optimize brightness and chlorine dioxide utilization. The initial pH was adjusted to maintain the final pH between 4.5 and 5.5 for optimal brightness. The pulp was used to create 2 brightness pads which were measured twice to provide the average brightness with standard deviations shown in Figure 4. Based on the data both pulps seemed to have a similar bleaching response and appear to be able to reach high brightness required for market pulps, although an additional brightening stage may be required. Some shives (probably from the nodes) were observed in the Moso pulp but should be easy to screen out in a larger operation.
[00452] The 1%> C102 pulps produced were utilized to make TAPPI standard handsheets to measure the physical properties. The results, shown in Figure 5, demonstrate that the Moso bamboo and poplar kraft pulps have quite similar properties with poplar responding better to refining with higher finenesses and tensile index.
[00453] The Moso fiber shows higher bulk and tear than the poplar which suggest a thicker cell wall fiber. This was confirmed through fiber morphology measurements shown in Table 4. The Moso fiber has slightly higher coarseness and length. Additionally, it shows a significantly higher fines content, as measured by the Fiber Quality Analyzer (FQA), than the hardwood poplar, which may explain the lower freeness values. Other studies have shown similar results of high fines in bamboo pulps and have suggested this may cause higher chemical consumption in some grades3' 9.
Table 4. Laboratory Cook Fiber Morphology
Coarseness1 AFL LWAFL Fiber/Gram LW Fines (mg/lOOm) (mm) (mm) (millions) (%)
Moso 11.8 (0.6)b 0.85 (0.03) 1.24 (0.03) 10.0 (0.3) 24.3 (2.1)
Poplar 8.1(0.5) 0.53 (0.02) 0.80 (0.02) 24.7 (0.3) 9.9 (1.2) aCoarseness measurements were made on samples with their fines removed
''Number in parentheses is the standard deviation of 3 tests
Example 41. Industrial Scale Experiments
[00454] Based on the results from the laboratory cooks of Example 40, a set of larger pilot scale cooks and bleaching runs were conducted on the Moso bamboo chips to confirm the pulpability and bleachability at a larger scale. Newer dried chips were cooked in four successive batches of approximately 85 kg of material in a 400L rotating digester equipped with indirect heating through a steam jacket. The cooks utilized an H-factor of 1100 and a max temp of 165°C. The white liquor consisted of an effective alkali of 17%, sulfidity 35%, and L/W ratio 4:1. The results for the four cooks are shown in Table 5. The results indicate improved delignification and yield over the laboratory cooks with an average kappa number of 17.2 and a yield of 46.5%. The resulting pulps were bleached with the bleaching sequence conducted as following:
• 02-stage, using 2.5% NaOH at 10% pulp consistency, 1 hour reaction at 110°C. The pulp was washed and resulted in a kappa number of 8.2.
• Do-stage, using a 0.2 kappa factor (0.64% charge of C102 ), a 1 hour reaction time, 10%) solids, and 50°C in a recirculating tower reactor after the initial pH was adjusted to 4 by the addition of sulfuric acid. The pulp was thoroughly washed and dewatered to approximately 20% consistency.
• E(P)-stage, using 2.5% NaOH and 0.5% H202, temperature 55°C, one hour reaction.
The final pH was measured to be 12.6°C.
• Di-stage, using 0.6% C102 charge at 70°C for 2 hours. No initial pH adjustment was utilized which resulted in a final pH of 6.4.
[00455] Samples of pulp were beaten in a PFI refiner and standard handsheets were evaluated for mechanical properties. The data was compared to the initial laboratory cooks
and is shown in Figure 6. Overall, the data indicates the materials responded similarly validating the laboratory results. The data suggests the pilot scale material has a slightly higher bonding as shown with higher initial tensile strength and lower bulk. Additionally the freeness was lower and it displayed lower tear strength at high refining levels. These results suggest the fiber in the pilot scale may have been overly processed as it performs if it were "pre-refmed". This could be due to either the mechanical action in the reactors or from the higher delignification in the initial cook.
[00456] To investigate the hypothesis that the pilot scale processing caused "pre-refming", fiber quality analysis (fines, fiber length, kink and curl) was performed on several samples throughout the pilot processing. Three samples were tested: (1) unbleached bamboo kraft pulp, (2) bamboo pulp after the O-stage and (3) final pulp after the D l -stage. The results, shown in Table 5, indicate the pulp fines are higher than the laboratory cooks and appear to increase with processing. Additionally the curl and kink of the pulp increase during the bleaching sequence. This confirms the hypothesis that this bamboo pulp may have undergone excessive mechanical damage during the pilot processing, which explains some of the differences noted from the laboratory cook.
Table 5. Pilot Cook Fiber Morphology
Unbleached Pulp 02-Stage Pulp Di-Stage Pulp
LWAa Fines (%) 28.75 27.58 30.12
LWA Fiber Length (mm) 0.985 1.010 0.898
LWA Curl Index 0.098 0.159 0.409
Kink Index (1/mm) 1.25 1.81 3.27
LWA (Length Weighted Average)
[00457] Fast growing biomass, such as bamboo, has the potential to serve an important role in the future of many industrial fields, such as pulp and paper industry. In this study an ECF bleached bamboo kraft pulp was compared to an ECF bleached poplar kraft pulp. Areas examined were pulpability, bleachability, and physical properties. Results indicate that the bamboo pulp cooked and bleached very similarly to a hardwood with similar yields and chemical requirements, had superior tensile properties at low refining, but had higher fines that lowered drainability as measured by Canadian Standard freeness.
[00458] Kraft pulping of Phyllostachys edulis chips can create an interesting non-wood pulp with yields and brightness similar to a Poplar hardwood pulps. Tensile properties appear to be slightly lower but the fibers have higher bulk and tear, presumably due to the higher coarseness fibers. The cooking and bleaching conditions are similar to poplar wood, which should allow for easy co-cooking of these materials, which could represent a convenient means of incorporating these materials into pulp and paper grades. Optimization of the cooking and bleaching parameters is essential for this pulp, and the data presented herein serve as a base for appropriate conditions. Example 42.
[0010] The following table provides fiber morphology for Moso bamboo samples run on Fiber Quality Analyzers, wherein not all of the data is from the same FAQ. Samples were produced according to the methods provided herein.
Table 6.
Example 43. Pulping of Pure Stand Bamboo Produced Via Micropropagation
[00459] A pure Phyllostachys Moso stand is produced according to the micropropagation methods of Examples 17, 19, 20, or 22. The propagated plants are transferred to a greenhouse or cultivated field to produce bamboo for biomass.
[00460] Biomass materials from the propagated pure Phyllostachys Moso stand are cooked and bleached, and used for pulp production according to the methods described in Examples 40, 41, and/or 42. As a comparison, the same amount of biomass materials from a natural Phyllostachys Moso bamboo population which is not a pure stand are used in the same pulp production process.
[00461] Quality of pulps produced by using the pure Phyllostachys Moso stand and the natural Phyllostachys Moso bamboo population are compared. Also compared are average cost/unit weight, energy consumed/ unit weight; average time spent/unit weight and other industrially important features of the two processes. Quality of pulps produced by using the pure Phyllostachys Moso stand are significantly better than the ones produced by using the natural Phyllostachys Moso bamboo population, and are produced with less cost, less energy spent, and/or less time.
Example 44.
[00462] A pure Phyllostachys Moso stand is produced according to the micropropagation methods described in Examples 17, 19, 20, or 22. The micropropagated plants are transferred to a greenhouse or cultivated field to raise mature bamboo plants. The pure stand is further propagated by natural or controlled pollination. Seeds from the pollinated pure stand are harvested and planted to raise more bamboo plants, and the resulted bamboo biomass materials are used for pulping, according to the methods described in Examples 40, 41, and/or 42.
Example 45.
[00463] A pure Phyllostachys Moso stand is produced according to the micropropagation methods described in Examples 17, 19, 20, or 22. The micropropagated plants are transferred to a greenhouse or cultivated field to raise mature bamboo plants. The mature bamboo plants or parts of the plants are used as explant for propagation in conventional methods, such as clump division (e.g., offsets planting and rhizome planting), whole culm cutting, layering, culm-segment cutting, branch cutting and macroproliferation. The propagated plants are used as biomass materials for pulping, according to the methods described in Examples 40, 41, and/or 42.
Example 46.
Materials and Methods
Bamboo samples
[00464] Phyllostachys edulis or "Moso" bamboo chip samples were acquired from Booshoot Gardens, LLC. The chips were screened with accepts set at 2mm and 8mm holes. The screened chips were air-dried and cold-stored prior to use.
Pulp production
[00465] Kraft pulping was completed using a 20L circulating digester with indirect heat exchanger following the conditions shown in Table 7. These conditions represent a fairly mild cook similar to those that would be utilized for a hardwood pulp like Eucalyptus. Higher sulfidity than typical was used following work done by Man Vu, Pakkanen, and Alen (Industrial Crops and Products 19 (2004) 49-57).
Table 7. Pulping Conditions
[00466] The resulting pulp was then bleached using a Quantum Rector following an OD(EP)D sequence utilizing the conditions shown in Table 8. The bleach sequence follows a typical sequence used for hardwood pulp.
Table 8. Bleaching Conditions.
[00467] Samples were well-washed with distilled water with no carryover. Elemental chlorine free chlorine dioxide was prepared through sulfuric acid acidification of sodium chlorite and collection of the chlorine dioxide gas into chilled water after passing through a sodium chlorite solution trap. Fiber morphology of coarseness and average fiber length was measured using an Optest Fiber Quality Analyzer. Physical properties were measured on
TAPPI Standard handsheets after they were refined in a PFI mill prepared following TAPPI Standards T-205 and T-248. Freeness, Caliper, Basis weight, Tensile, Tensile Energy Adsorbed, Tear, and Brightness were measured utilizing TAPPI Standards T220, T411, T414, and T525.
Results
Bamboo pulp properties
[00468] The resulting pulps were characterized for morphology to understand their usefulness as a pulp raw material. The results, shown in Table 9, indicate that the bamboo fiber were between a hardwood and softwood fiber with Length Weighted Average Fiber Lengths, LWAFL, of approximately 1.0 mm for samples with fines, and around 1.4mm for samples with the fines removed using a 0.004" screen. LWAFL is the metric typically used when predicting fiber performance. Arithmetic Average Fiber Length, AAFL, was also provided for the two data sets, which demonstrates the original sample to have a high amount of fines.
Table 9. Fiber morphology.
Note: AAFL is Arithmetic Average Fiber Length and LWAFL is Length Weighted Average Fiber Length
[00469] The fiber morphology indicates that the bamboo pulp to have fairly high fines content, which would need to be removed to be useful as a replacement for wood based pulp. Fines material, defined as material below 0.2mm in length have a high surface area which consume polymeric strength and processing aids, but do not contribute much to the network strength of the paper web. Additionally, high fines can cause papers to become overly stiff. On a positive note the high amount of surfaces present from the fines could improve the optical properties such as opacity.
[00470] Measuring the mass loss between the two samples indicates the material loss to create a relatively fines free sample indicated a 19.3% loss on a mass basis. This loss in material represents a likely yield drop from the original 45% to approximately 35%, which would represent a hefty increase to the cost of manufacture. Considering just the variable cost for most pulps to be around $400/metric ton (dry basis) - you have increase your costs by approximately 100/metric ton with this loss. It is possible that some grades of paper would not require the fines to be removed, but this would likely classify this pulp as a value grade discounting the price $100 to 200/MT.
[00471] Methods to make up for this loss would likely need to be in a significantly lower cost of biomass as the pulping and bleaching conditions are similar to a hardwood pulp, like Populus or Eucalyptus. However, when considering wood cost to range from $100 to $150/metric ton (dry basis) for most mills the bamboo pulp would need to be almost zero cost. The other alternative is to find a bamboo pulp or process that does not generate the significant amount of fines. It is recommended that pulp development efforts be concentrated in this area if further work is desired.
Physical and optical properties ofhandsheets
[00472] The bleached pulps were refined in a PFI mill at 100, 1000, and 2000 revolutions and utilized to prepare handsheets to measure their physical and optical properties, shown in Table 9. Additionally the properties are plotted and shown in Figure 7. The results indicate the fiber to develop strength properties similar to a traditional wood pulp but to have a fairly significant drop in freeness with refining, presumably due to the high fines content.
[00473] The brightness properties were slightly lower than one would want for a premium market pulp, with an average brightness of 87 ISO brightness and a typical target of 90. The pulps do show high bleachability response, requiring relatively low bleaching chemicals, especially for a nonwood fiber, which typically struggle to get high brightnesses. An additional bleach stage or higher chlorine dioxide charges would likely bring the brightness up to a 90 brightness. Additionally, screening of the bamboo nodes after pulping would be recommended as it is likely these high lignin portions of the plant contribute to higher bleach consumption.
[00474] Inspecting the drainage properties of the pulps through the Canadian Standard Freeness value it can be seen that the drainage is reduced fairly quickly with refining. The initial value of 590mL was slightly lower than unrefined wood pulps but as the pulp had not been dried this was not unexpected. The high fines content was expected to lower the pulp
drainage, but did not appear to be too detrimental with the low levels of refining suggesting the fines to have high aspect ratios. The freeness drop with refining was fairly high suggesting once again thin walled fibers that were not robust enough to take significant energy without hydrating and fibrillation. This is a positive as the fibers are easily refined but a negative as the freeness drop required to generate strength would likely cause a reduction in paper making speed for most grades. The density properties of caliper and condition bulk were in the typical range for kraft pulps with these strengths and decreased as expected with refining due to higher fiber collapse and increased bonding area.
[00475] Looking at just the strength properties of tensile index and tear, which represent the network and individual fiber strength respectively, one can see refining quickly develops the strength indicating the fibers are easily fibrillated and hydrated but do not appear to have significant defects. The strength quickly tops out with a gain of only 10 Nm/g for the last 1000 revs of refining suggesting the fibers to have thin cell walls and possibly have some pre- refming due to the mixing action in the bleaching steps and the lack of hornification from never being dried. This is not as critical as the overall strength being a bit on the low side if it were to be considered for applications replacing softwood fibers, which typically can top 85 Nm/g at the leveling off of the strength at the highest refining points. On a positive side the strengths were quite high if replacing a hardwood which typically level out around a 50 Nm/g tensile index. It is probable the fiber length of the species is a dominant factor in determining the network strength. Although the fiber length can be affected by age, it is likely difficult to change significantly.
Table 10. Handsheet physical and optical properties.
[00476] Kraft pulping of Phyllostachys edulis chips created an interesting non-wood pulp that has properties in-between bleached Eucalyptus kraft and northern softwood kraft pulps. This property difference is supported by the fiber morphology which indicate lengths and coarsenesses in between these two grades as well falling closest to an bleached Aspen grade. Brightness results indicate the pulp should be easily bleached in production and able to achieve typical market brightness with some optimization and screening. The high fines content of the pulp was the largest area that will need to developed, with additional processing required on either the pulp or the bamboo biomass.
[00477] Unless otherwise indicated, all numbers expressing quantities of ingredients, properties such as molecular weight, reaction conditions, and so forth used in the specification and claims are to be understood as being modified in all instances by the term "about." Accordingly, unless indicated to the contrary, the numerical parameters set forth in the specification and attached claims are approximations that may vary depending upon the desired properties sought to be obtained by the present invention. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques.
[00478] Notwithstanding that the numerical ranges and parameters setting forth the broad scope of the invention are approximations, the numerical values set forth in the specific examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in their respective testing measurements.
[00479] Recitation of ranges of values herein is merely intended to serve as a shorthand method of referring individually to each separate value falling within the range. Unless otherwise indicated herein, each individual value is incorporated into the specification as if it were individually recited herein. All methods described herein can be performed in any suitable order unless otherwise indicated herein or otherwise clearly contradicted by context. The use of any and all examples, or exemplary language (e.g., "such as") provided herein is intended merely to better illuminate the invention and does not pose a limitation on the scope of the invention otherwise claimed. No language in the specification should be construed as indicating any non-claimed element essential to the practice of the invention.
[00480] Groupings of alternative elements or embodiments of the invention disclosed herein are not to be construed as limitations. Each group member may be referred to and claimed individually or in any combination with other members of the group or other elements found herein. It is anticipated that one or more members of a group may be included in, or deleted from, a group for reasons of convenience and/or patentability. When any such inclusion or deletion occurs, the specification is deemed to contain the group as modified thus fulfilling the written description of all Markush groups used in the appended claims.
[00481] Certain embodiments of this invention are described herein, including the best mode known to the inventors for carrying out the invention. Of course, variations on these described embodiments will become apparent to those of ordinary skill in the art upon reading the foregoing description. The inventor expects skilled artisans to employ such variations as appropriate, and the inventors intend for the invention to be practiced otherwise than specifically described herein. Accordingly, this invention includes all modifications and equivalents of the subject matter recited in the claims appended hereto as permitted by applicable law. Moreover, any combination of the above-described elements in all possible variations thereof is encompassed by the invention unless otherwise indicated herein or otherwise clearly contradicted by context.
[00482] Specific embodiments disclosed herein may be further limited in the claims using consisting of or consisting essentially of language. When used in the claims, whether as filed or added per amendment, the transition term "consisting of excludes any element, step, or ingredient not specified in the claims. The transition term "consisting essentially of limits the scope of a claim to the specified materials or steps and those that do not materially affect the basic and novel characteristic(s). Embodiments of the invention so claimed are inherently or expressly described and enabled herein.
[00483] In closing, it is to be understood that the embodiments of the invention disclosed herein are illustrative of the principles of the present invention. Other modifications that may be employed are within the scope of the invention. Thus, by way of example, but not of limitation, alternative configurations of the present invention may be utilized in accordance with the teachings herein. Accordingly, the present invention is not limited to that precisely as shown and described.
[00484] Unless defined otherwise, all technical and scientific terms herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. Although any methods and materials, similar or equivalent to those described
herein, can be used in the practice or testing of the present invention, the preferred methods and materials are described herein.
[00485] All publications, patents, and patent publications cited are incorporated by reference herein in their entirety for all purposes.
[00486] The publications discussed herein are provided solely for their disclosure prior to the filing date of the present application. Nothing herein is to be construed as an admission that the present invention is not entitled to antedate such publication by virtue of prior invention.
[00487] While the invention has been described in connection with specific embodiments thereof, it will be understood that it is capable of further modifications and this application is intended to cover any variations, uses, or adaptations of the invention following, in general, the principles of the invention and including such departures from the present disclosure as come within known or customary practice within the art to which the invention pertains and as may be applied to the essential features hereinbefore set forth and as follows in the scope of the appended claims.
REFERENCES
1. Liese, W., Research on bamboo. Wood Science and Technology 1987, 21, (3), 189- 209.
2. Scurlock, J. M. O.; Dayton, D. C; Hames, B., Bamboo: an overlooked biomass resource? Biomass and Bioenergy 2000, 19, (4), 229-244. 3. Zhao, G.; Lai, R.; He, B.; Greschik, T.; Li, X., Replacement of Softwood Kraft Pulp with ECF-Bleached Bamboo Kraft Pulp in Fine Paper. BioResources 2010, 5, (3), 1733-1744.
4. Lee, A. W. C; Xuesong, B.; Peralta, P. N., Selected physical and mechanical properties of giant timber bamboo grown in South Carolina. Forest Products Society: Madison, WI, USA, 1994; Vol. 44.
5. Long, S. P.; Jones, M. B.; Roberts, M. J., Primary productivity of grass ecosystems of the tropics and sub-tropics / edited by Stephen P. Long, Michael B. Jones, Michael J. Roberts. Chapman & Hall: London ; New York :, 1992.
6. Man Vu, T. H.; Pakkanen, H.; Alen, R., Delignification of bamboo (Bambusa procera acher): Part 1. Kraft pulping and the subsequent oxygen delignification to pulp with a low kappa number. Industrial Crops and Products 2004, 19, (1), 49-57.
7. Salmela, M.; Alen, R.; Vu, M. T. H., Description of kraft cooking and oxygen-alkali delignification of bamboo by pulp and dissolving material analysis. Industrial Crops and Products 2008, 28, (1), 47-55. 8. Dence, C. W.; Reeve, D. W., Pulp bleaching: Principles and practice. TAPPI Press: Atlanta, Ga, 1996.
9. Wai, N. N.; Nanko, H.; Murakami, K., A morphological study on the behavior of bamboo pulp fibers in the beating process. Wood Science and Technology 1985, 19, (3), 211- 222.