Abstract
Here, we compared the polymorphism among 13 Avena species revealed by the iPBS markers and soluble carbohydrate profiles in seeds. The application of seven iPBS markers generated 83 bands, out of which 20.5% were polymorphic. No species-specific bands were scored. Shannon’s information index (I) and expected heterozygosity (He) revealed low genetic diversity, with the highest values observed for A. nuda (I = 0.099; He = 0.068). UPGMA clustering of studied Avena accessions and PCoA results showed that the polyploidy level is the main grouping criterion. High-resolution gas chromatography revealed that the studied Avena accessions share the same composition of soluble carbohydrates, but significant differences in the content of total (5.30–22.38 mg g−1 of dry weight) and particular sugars among studied samples were observed. Sucrose appeared as the most abundant sugar (mean 61.52% of total soluble carbohydrates), followed by raffinose family oligosaccharides (31.23%), myo-inositol and its galactosides (6.16%), and monosaccharides (1.09%). The pattern of interspecific variation in soluble carbohydrates, showed by PCA, was convergent to that revealed by iPBS markers. Thus, both methods appeared as a source of valuable data useful in the characterization of Avena resources or in the discussion on the evolution of this genus.
Similar content being viewed by others
Introduction
The genus Avena L. (Poaceae), which can be found within the Aveneae tribe, is represented by about 30 species (Baum 1977). The majority of oats represent wild and weedy species, but there are also crop species among them—Avena strigosa Schreb., A. abyssinica Hochst., A. byzantina C.K., and A. sativa L. A. sativa is the most commonly cultivated oat used for animal feed, human consumption, and industry purposes (Boczkowska and Tarczyk 2013). All representatives of the genus Avena are self-pollinated annuals, with the exception of A. macrostachya which is an outbreeding perennial (Katsiotis et al. 1997). Genus Avena contains diploid (AA and CC), tetraploid (AACC and AABB), and hexaploid (AACCDD) species with a basic chromosome number of seven (Baum 1977, Loskutov and Rines 2011). Surprisingly, there is no information about diploid species with BB or DD genome, although A. canariensis was postulated as a candidate DD genome (Yan et al. 2016). Cytogenetic analyses revealed that the D genome and B genome are highly similar to the A genome type (Rajharthy and Thomas 1974; Katsiotis et al. 1997; Tomaszewska et al. 2022). Detailed cytological studies showed that species within the genus Avena share their genome(s) with one another, which proves their evolutionary relationships (Linares et al. 1998). Moreover, the evolution of the oat nuclear genomes appeared as a complex process which involved divergence at the diploid level from a common diploid ancestor and then convergence, followed by divergence, at the polyploid level (Thomas 1992).
Oat grains are known for their high level of calcium, β-glucan soluble fiber (Andon and Anderson 2008; Jenkins et al. 2002; Yarnell and Abascal 2001a, 2001b), and high-quality oil and protein content (Fardet 2010; Peterson 2001). Furthermore, they are rich in antioxidants as well as anti-inflammatory and antiatherogenic compounds (Daou and Zhang 2012). As a result, oats have attracted the attention of breeders. However, despite intensive efforts which have been made to describe the genetic diversity and perform biochemical characteristics of particular collections, representing existing gene pools of cultivated oats, we are still lacking their comprehensive characterization. Currently, available literature shows that many wild and weedy Avena species can be used in breeding programs as donors of many valuable traits which enable the increase of the yield and grain quality (e.g., A. fatua, A. sterilis, A. ludovicina, A. occidentalis; Trofimovskaya et al., 1976; Frey 1991; Miller et al. 1993; Loskutov et al. 2021) as well as protein content in straw and green matter (e.g., A. abyssinica, A. magna, A. ludovicina, A. sterilis; Mal 1987; Rezai 1977; Loskutov and Rines 2011; Ociepa 2019). Wild Avena species can be used also to improve the cultivated forms of oat in terms of disease and pathogen resistance, earliness, or highly productive tillering (Marshal and Shaner 1992; Leonard et al. 2004; Okoń et al. 2016, 2018; Gordon et al. 2022). The lack of precise characterization of the genetic resources is the main obstacle to successful and efficient breeding programs. The knowledge about the genetic diversity presented in cultivated oats and their wild relatives, information about relationships among these species, and their detailed biochemical characteristic is crucial not only for utilization of the rich genetic diversity of wild Avena species (Govindaraj et al. 2015) but also for conservation of this valuable genetic resources (Leggett 1996).
One of the special characteristic features of the eucaryotic genomes is the presence of repetitive elements which are present in a high number of copies scattered throughout the genome. These sequences can be identified as tandemly repeated elements (e.g., microsatellites) or transposable elements (TEs). The discovery of the TEs change our way of thinking about the mechanisms responsible for genome diversification and evolution (McClintock 1984; Kunze et al. 1997). TEs, due to their ability to replication and change their location in the genome, can induce various types of mutations beginning with insertion to protein-coding genes, illegitimate recombinations, and genomic rearrangements, up to chromosomal breakage (Feschotte et al. 2002; Feschotte and Pritham 2007; Wessler 2006; Almojil et al. 2021). The TEs activity has also an influence on gene expression as their insertion within the sequence of the particular gene not only may destroy the gene structure and prevent its transcription but also may alter the gene expression profile by insertion into its promoter region (Kumar and Bennetzen 1999; Levin and Moran 2011; Bourque et al. 2018). Based on the structure and mechanisms of transposition, TE is divided into two classes: class I which contains retrotransposons (retro-TEs) and class II which includes DNA transposons (DNA-TEs). Retrotransposons, which are “copy and paste” elements, appear as a very important fraction of the higher-plant genomes. For example, in maize (Zea mays), TEs constitute for 84.2% of its genome (including 75.6% of retro-TEs and 8.6% of DNA-TEs), in wheat (Triticum aestivum) for 79.8% of the genome (63.7% of retro-TEs and 14.9% of DNA-TEs), whereas in tomato (Solanum lycopersicum) for 63.2% of the whole genome (62.3% of retro-TEs and 0.9% of DNA-TEs) (Bonchev 2016). As a consequence retrotransposons activity is presented as one of the major factors responsible for the expansion of plant genomes (Kumar and Bennetzen 1999).
Although there were a number of studies on genetic diversity and differentiation as well as the evolution of species representing genus Avena, TEs were poorly studied so far in this group of plants. However, based on the analyses of available sequencing data, it is known that repetitive elements (LTR retrotransposons make up most of the classified elements) may comprise even 83% of the Avena genomes (Maughan et al. 2019). Moreover, few studies can be found which were devoted to the identification and characterization of TEs in Avena strigosa, A. sativa, A. sterilis, A. vaviloviana, A clauda, and A. magna (Tomás et al. 2016; Linares et al. 1999; Katsiotis et al. 1996). Retrotransposons have proved their high suitability for the development of many DNA marker systems like SSAP (Waugh et al. 1997), RBIP (Flavell et al. 1998), IRAP, and REMAP (Kalendar et al. 1999) or iPBS (Kalendar et al. 2010), which appeared as highly effective molecular tools with a number of applications in plant genetics and breeding (Kalendar et al. 2018). Unfortunately, their application in genetic studies on Avena species is very limited, with few papers reporting the application of REMAP for comparative analysis of selected Avena species (Tomás et al. 2016; Paczos-Grzęda and Bednarek 2014) or joint AFLP and SSAP markers application for construction of a genetic map of diploid Avena (Yu and Wise 2000).
The main limitation of all DNA marker techniques which are based on retrotransposons is the need for sequence information to develop specific PCR primers, allowing the amplification of unique, retrotransposon-related fragments. This fact hampers the exploitation of the above-mentioned molecular techniques by making it available generally for the model organisms or utilized crops for which genomic data are available. Fortunately, a new retrotransposon-based DNA technique was developed, called iPBS (Kalendar et al. 2010), which exploited the conserved region of the primer binding site (PBS) of LTR retrotransposons, which is directly involved in the initiation of the reverse transcription during the replication cycle of retrotransposon. In contrast to other retrotransposon-based molecular markers that require specific primers for the flanking regions of the particular retrotransposon, iPBS uses universal primers which enable the amplification of various types of LTRs. So far, there are several studies in which dominant iPBS markers were successfully applied for the estimation of genetic diversity and differentiation of plants (Bonchev and Vassilevska-Ivanova 2020; Khapilina et al. 2021; Naeem et al. 2021) and fungi (Ates et al. 2019; Turzhanova et al. 2020; Erper et al. 2021). According to our knowledge, iPBS markers have only been used once for the genetic study of genus Avena, reporting genetic diversity and differentiation of Latvian A. fatua populations (Nečajeva et al. 2021).
In the present study, retrotransposon-based iPBS markers were used for the first time to assess DNA polymorphism and genetic relationships between 13 Avena species. In our research, four levels of organization of studied material were applied to establish mutual relationships between studied Avena accessions: species level, the taxonomic system of species within genus Avena according to Baum (1977), ploidy, and genome type. Biochemical characteristics of the seeds, for example, the composition and content of soluble carbohydrates (mainly raffinose family oligosaccharides, RFOs), are also commonly used for the estimation of intra- and inter-specific diversity. The suitability of that approach was demonstrated for the species representing the genus Lupinus (Piotrowicz-Cieslak 2005), Vicia (Lahuta et al. 2018; Lahuta et al. 2020), Lathyrus (Ibrahim et al. 2021), Agave, Dasylirion (Mancilla-Margalli and Lopez 2006), and for Vitis vinifera L. varieties (Bhouri et al. 2016) or willow cultivars (Budny et al. 2021). Oat grains are also abundant in sugars and oligosaccharides (MacLeod and McCorquodale 1958; Kaur et al. 2019). Therefore, we made an attempt to determine the usefulness of intra- and inter-specific variation in soluble carbohydrate profiles in caryopses for the characterization of the 13 Avena species. In our project, we decided to combine both approaches to characterize the selected Avena accessions and to compare the revealed polymorphism
Materials and methods
Material
The research material consisted of caryopses of 13 species of the genus Avena, represented by 1–9 different, randomly selected accessions, that we were able to obtain from the National Centre for Plant Genetic Resources (NCPGR) of the Plant Breeding and Acclimatization Institute in Radzików, Poland; Nordic Gene Bank (NGB) and N.I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR). The list of all 60 tested Avena accessions is provided in Table S1.
DNA extraction and iPBS genotyping
DNA extraction was performed with the use of the CTAB method by Murray and Thompson (1980) with some modifications (Polok, 2007). For DNA extraction, pooled (bulked) samples were applied in which particular Avena accession was represented by an equal amount of fresh tissue collected from 15 randomly selected individuals grown from seeds in a greenhouse of the Department of Plant Physiology, Genetics and Biotechnology at the University of Warmia and Mazury in Olsztyn, Poland. Young, healthy leaves were collected, cleaned, and stored at −20 °C until DNA extraction. DNA quality was verified by agarose gel electrophoresis, and its concentration was determined spectrophotometrically using NanoDrop (ND-1000 UV/Vis).
Based on the results of initial tests of 34 PBS primers (Kalendar et al. 2010), a set of seven primers that gave polymorphic, clearly identifiable, and repeatable bands were selected for further analyses (Table 1). The initial tests and optimalisation of PCR conditions for studied Avena species were conducted according to the procedure described in Milarska et al. (2022). The reproducibility of band profiles for each of the selected PBS primers was verified. This verification was based on a comparison of the electrophoretic profiles for selected eleven Avena accessions. Data were generated and compared in two replicates. Gels were then checked to identify iPBS amplicons (bands) in one or both replicates. Amplification was performed according to the procedure described by Kalendar et al. (2010) with modifications (Androsiuk et al. 2015). The PCR products were then analyzed by horizontal electrophoresis (1 × TBE buffer at 100 V for 2 h) in 1.5% agarose gel stained with 0.5 mg/ml ethidium bromide.
Genetic diversity analyses
All bands that could be reliably read across all Avena accessions were scored as either present (1) or absent (0) and treated as single dominant loci. The obtained binary matrix (Table S2) was subjected to further analyses and the following parameters were estimated: total number of bands per primer (NB), percentage of polymorphic bands (P), Shannon’s information index (I), expected heterozygosity (He), genetic similarity for each pair of accessions according to Dice (1945), as well as standard Nei’s genetic distance and identity (Nei 1972; Nei 1978) for each pair of analyzed species or genome types. Dice similarity values were then used for grouping analysis of Avena accessions using UPGMA clustering implemented in PAST 3.18 (Hammer et al. 2001). The matrix containing Nei’s genetic distance values was used to perform the principal coordinate analysis (PCoA) in GenAlEx 6.5 (Peakall and Smouse 2006, 2012) to assess the genetic associations of the Avena species and also for UPGMA clustering to estimate relationships between Avena genome types. Moreover, the value of polymorphic information content (PIC) for each marker was estimated using the formula described by Roldan-Ruiz et al. (2000).
Finally, the obtained data was also subjected to molecular variance analysis (AMOVA). Analysis of molecular variance was performed with Arlequin 3.5. For this analysis, the iPBS data was treated as haplotypic, comprising a combination of alleles at one or several loci (Excoffier et al. 2005). The significance of the fixation indices was tested using a non-parametric permutation approach, the method implemented in Arlequin 3.5 (Excoffier et al. 1992; Excoffier et al. 2005). The analysis included the partition of the total iPBS variation between Avena species not only into within- and among-species variation components, but also with an application of additional structuring of the data which encompassed Avena sections according to Baum (1977), ploidy, and genome types. Furthermore, average gene diversity over loci (πn) was also estimated for specific members of these four groups, according to Tajima (1983) and Nei (1987) model implemented in Arlequin 3.5.
Analysis of the soluble carbohydrates
The analysis of the soluble carbohydrates profiles was carried out on a group of 47 selected accessions representing 13 species of the genus Avena (Table S1). The research material consisted of ripe, husked Avena caryopses, which were grounded in a ball mill homogenizer (22 Hz for 2 min). Samples with a weight of 40 mg were prepared in three replicates for each accession. Extraction of carbohydrates was carried out in a mixture of ethanol and water (1: 1, v/v), containing xylitol as an internal standard, at 90 °C for 30 min. The qualitative and quantitative analyses of the soluble carbohydrates were performed using the high-resolution gas chromatography method, as described earlier (Lahuta 2006).
The obtained data were statistically analyzed with the use of Statistica v.12 software (StatSoft Inc., Tulsa, OK, USA). The significance of differences between mean values was determined by a one-way analysis of variance. A post-hoc analysis using Fisher’s least significant difference (LSD) test was carried out to compare the means. Differences were considered to be significant when p < 0.05. Moreover, principal component analysis (PCA) was used to visualize the structure of relationships between the studied Avena species. PCA included eight variables (fructose, glucose, sucrose, raffinose, stachyose, myo-inositol, galactinol, and di-galactosyl myo-inositol).
Results
Genetic variation
Genetic analysis of 60 Avena accessions with the use of seven PBS primers identified 83 amplification products (bands). The highest number of bands (21) was revealed by PBS 2378 primer, whereas the lowest number (7) was scored for PBS 2376. The average number of bands per primer was 11.85. Out of all identified loci, across all genotypes, 17 (20.5%) were polymorphic (Table 1, File S1). No species-specific bands of possible diagnostic application were scored. Only in the case of one locus (2253/4) its quasi-diagnostic character was observed, as no amplification product was scored for any tested accessions representing two Avena species: A. atlantica and A. longiglumis (Table S2). The set of PBS primers used in this study generated 13 highly informative loci with PIC value greater than 0.43 (data not shown) which represented 76.47% of polymorphic loci. The average PIC for the particular primer range from 0.04 (PBS 2229) to 0.21 (PBS 2376) with an average value of 0.10.
iPBS markers enabled the detection of polymorphism not only between the tested Avena species but also between the majority of accessions representing individual species. The number of iPBS bands ranged from 74 for the A. insulanis and A. sativa, to 82 for A. abyssynica, A. fatua, and A. nuda. The highest rate of polymorphic bands was scored for accessions representing A. fatua (18.07%) and A. nuda (16.87%), whereas the lowest number of polymorphic bands was observed for A. hirtula and A. sativa (1.20%). In order to estimate the genetic diversity of studied Avena species, Shannon’s information index (I) and expected heterozygosity (He) were calculated. Both parameters revealed a low level of gene diversity, with the highest values observed for A. nuda (I = 0.099; He = 0.068), A. abyssinica (I = 0.091; He = 0.063), and A. fatua (I = 0.093; He = 0.062). No polymorphism was scored among accessions representing A. atlantica, A. longiglumis, A. insulanis, and A. sterilis (Table 2).
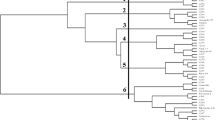
Values of Dice similarity coefficient estimated between all pairs of accessions (Table S3) suggested low genetic differentiation within studied material, as the minimum value of that coefficient was 0.893, which described the relation between A atlantica and seven out of nine A. byzantine accessions and between A hirtula 51847 (IHAR) and A. sativa 52267 (IHAR). The UPGMA clustering of accessions based on the Dice similarity coefficient was performed and based on the results a cluster tree was plotted (Fig. S1). The analyzed Avena accessions were clustered generally according to their species name. Furthermore, two clusters could be distinguished in the presented tree. Cluster I consisted of 25 accessions representing the following Avena species: A. strigosa (6), A. longiglumis (1), A. atlantica (2), A. hirtula (3), A. damascena (5), A. nuda (2), A. abyssynica (3), A. barbata (2), and A. fatua (1). Cluster II gathered 35 accessions representing A. abyssynica (3), A. nuda (2), A. barbata (2), A. sterilis (7), A. fatua (4), A. insulanis (2), A. byzantiana (9), and A. sativa (6). Based on the polymorphism revealed by iPBS markers, a matrix of Nei’s genetic distances (DN) between analyzed Avena species was also created and subjected to Principal coordinate analysis (PCoA). The Nei’s genetic distance values ranged from 0.010 (A. sativa vs. A. byzantiana) to 0.199 (A. atlantica vs. A. byzantiana). The results of PCoA revealed that 85.88% of the genetic variability of the analyzed species was explained by its first three components (68.17%, 11.31%, and 6.4%, respectively). Figure 1 illustrates the dispersion of the analyzed Avena species on the plane defined by the first two axes. The results of PCoA distinguished three groups of species clearly dispersed along the Coord.1. The first group included A. atlantica, A. longiglumis, A. strigosa, A. hirtula, and A. damascena. In turn, A. sativa, A. sterilis, A. fatua, A. byzantina, and A. insularis formed the second group. In the central part of the graph, one could find A. barbata, A. nuda, and A. abyssynica. In order to estimate the relationships between Avena genome types, individuals were first grouped according to the defined genome type, and then Nei genetic distance (DN) values between these groups were estimated. Obtained DN values ranged from 0.019 (As vs. AB genome) up to 0.148 (Al vs. ACD) and 0.170 (Al vs. AC). The results of UPGMA clustering based on DN values, divided the studied Avena genome types into three clusters. Al genome appeared as the most distinct, solitary cluster. The second cluster gathered Ad, As, and AB genomes, whereas the third cluster consisted of AC and ACD genomes (Fig. 2).
Genetic association of six genome types of Avena based on iPBS data. Genome types and their assignation to particular Avena species according to the data located in Table 2
The results of AMOVA showed that most of the detected variability (66.68%) was located between the analyzed Avena species, while the remaining 33.32% determined the diversity between the accessions representing individual species (Table 3). In the case when the sections of Avena species proposed by Baum (1977) were considered, 35.56% of the variation was found among sections, 30.29% of the total variation resided among species within sections, whereas the remaining 34.14% was present within individual species. According to the values of average gene diversity over loci, the most diverse section was Ethiopica, followed by Agraria, Tenuicarpa, Avena, and Pachycarpa (Table 4). For the latter, only one Avena species (A. insulanis) represented that section in our study, and no polymorphism was found among A. insulanis accessions, thus πn was 0. For the ploidy level of Avena species, the highest proportion of the total variation was found among groups differed in terms of ploidy level (47.88%), whereas variation among species within these groups and within species was 23.70% and 28.42%, respectively (Table 3). Estimated πn values showed that tetraploid species were the most differentiated and were followed by diploid and hexaploidy species (Table 4). Finally, when the genome types of Avena species were considered almost half (49.02%) of the total variation was found among these types, whereas 21.52% was present among species within these groups and 29.45% resided within species (Table 3). The πn values estimated for each genome type revealed that the most diverse genome type was AB, followed by As, ACD, Ad, AC, and Al (Table 4). For AC and Al genome types, πn values were 0.0 due to the fact that in our study, only one Avena species (A. longiglumis) represented by one accession represented the Al genome type, whereas in the case of AC genome type, only one Avena species (A. insulanis) represented that genome type, and no polymorphism was found among accessions representing that species.
Soluble carbohydrates
In caryopses of all studied Avena species, the following soluble carbohydrates were identified: sucrose, raffinose family oligosaccharides (RFOs, represented by raffinose and stachyose), myo-inositol, and its a-d-galactosides (galactinol and digalactosyl myo-inositol), fructose and glucose. Despite the identical qualitative composition, the analyzed oat species revealed high variation in the content of soluble carbohydrates, both among accessions representing each species (Table S4, Fig. S2) as well as between species (Fig. 3, Table 5). The content of total soluble carbohydrates ranged from 6.54 ± 1.43 to 18.28 ± 1.00 mg g−1 of dry weight (DW), in A. sterilis and A. atlantica, respectively. Sucrose, the major sugar (Fig. 3), accounted for more than 50% of total carbohydrates in most oats species/accessions. The highest content of sucrose (ca 8–10 mg g−1 DW) was found in caryopses of A. atlantica, A. abyssinica, and A. sativa, whereas the lowest (ca 3–4 mg g−1 DW) in A. sterilis and A. insulanis (Fig. 3). The next to sucrose was raffinose and stachyose, occurring together at a level as high as sucrose (in A. insulanis), slightly lower (in A. strigosa, A. hirtula, and A. longiglumis), or even 3–4-fold lower (in A. abyssinica, A. byzantina, A. fatua, and A. nuda, Fig. 3). Regardless of the species, raffinose dominated above stachyose (except for 6 among 47 accessions; Table S4). Myo-inositol and its galactosides were less abundant carbohydrates (0.28–1.44 mg g−1 DW), sharing only a few % of total soluble carbohydrates in most species (Table S4). Monosaccharides accounted for no more than 1% of all soluble carbohydrates, with except for A. abyssinica (1.12%), A. nuda (1.19%), and A. byzantina (2.3%).
The results of the principal components analysis (PCA) indicated that 86.75% of the variability in the content of soluble carbohydrates in the analyzed species is explained by its first three components (44.03%, 27.55%, and 15.17%, respectively). The content of RFOs (raffinose and stachyose) as well as galactinol and di-galactosyl myo-inositol had the greatest influence on the dispersion of the analyzed samples against PC1. In the case of PC2, these were glucose, fructose, and sucrose (given in descending order), whereas in the case of PC3 myo-inositol had a decisive role in discriminating of the analyzed oat accessions. The projection of analyzed Avena species on the first two axes was shown in Fig. 4. The diploid Avena species (black) were scattered mainly on the left from PC1 and in the central part of Fig. 4 (with A. atlantica diverged along the PC1). The hexaploid Avena species marked with red color (A. sativa, A. byzantina, A. fatua, and A. sterilis) could be found in the peripheral areas of the plane defined by PC1 and PC2 axes, with A. byzantina that diverged along PC1. The tetraploid Avena species (A. abyssynica, A. barbata, and A. insulanis, marked with green color) occupied the area between the two mentioned above groups of species.
Discussion
For centuries, oat, which served mainly as forage and less frequently as food, was treated as a minor cereal which was cultivated predominantly on poor soils unsuitable for the cultivation of other cereals (Moore-Colyer 1995). Currently, the oat is the sixth most cultivated cereal in the world; however, recently, there has been observed an increase in interest in oat as a trendy, healthy functional food (Hofmann 1995; Andersson and Börjesdotter 2011). The growing interest in oat revealed the need for intensification of breeding programs which aimed at providing new varieties characterized by higher yield, improved biochemical composition of grain, increased resistance to pests and/or abiotic stresses, etc.
Comprehensive characteristics of breeding material are a crucial element for the planning of effective and efficient breeding programs as it allows precise evaluation of genetic variation available to breeders (Govindaraj et al. 2015). Although the evaluation of genetic variation in oat is less intensively studied in comparison with other cereal crops, there are a number of papers describing genetic diversity and differentiation of various Avena species and accessions. Initially, the polymorphism of several isoenzyme systems was used for the genetic characteristic of Avena (Marshall and Allard 1970; Craig et al. 1972; de la Hoz and Fominaya 1989). Later on, different DNA marker systems were used for the investigation of polymorphism in oats: restriction fragment length polymorphism (RFLP) (Beer et al. 1993; Nocelli et al. 1999), random amplified polymorphic DNA (RAPD) (Heun et al. 1994; Drossou et al. 2004), amplified fragment length polymorphism (AFLP) (Fu and Williams 2008; Constandinou et al. 2018), inter simple sequence repeats (ISSR) (Boczkowska and Tarczyk 2013; Sharma et al. 2016), or microsatellite markers (SSR) (Li et al. 2000a; Nikoloudakis et al. 2016). Application of these techniques allowed also genetic mapping (O’Donoughue et al. 1992; Portyanko et al. 2001), resolving the questions of phylogeny and systematic position of species (Alicchio et al. 1995; Li et al. 2000b), and identification of molecular markers associated with certain traits, which were then used for fast and efficient selection of breeding material (Rines et al. 2006). One of the available genetic marker system which is increasingly used in plant genetics and breeding is iPBS (Kalendar et al. 2010). So far, this molecular technique was successfully applied in genetic studies of, e.g., guava (Mehmood et al. 2016), potato (Demirel et al. 2018), barley (Bonchev et al. 2019), oregano (Karagoz et al. 2020), peach, and nectarine (Naeem et al. 2021). TEs, due to their mobile nature, had a significant impact on the organization, plasticity, and evolution of genomes (Frost et al. 2005). Therefore, the retrotransposon-based iPBS markers appeared as an ideal candidate molecular tool suitable for genetic diversity and evolutionary studies of genus Avena, which although extensively studied still remain elusive (Fu 2018).
Genetic diversity revealed by iPBS markers
In the current paper, iPBS markers proved their usefulness in genetic studies of the genus Avena. Application of that retrotransposon-based molecular technique not only allowed to trace genetic variation among studied Avena accessions but also provided valuable data which may become useful in the comprehensive study of the evolution of the whole genus. iPBS genotyping of our Avena collection revealed that 20.5% of amplified fragments (bands) were polymorphic. The highest polymorphism was found among accessions representing A. fatua (18.07%) and A. nuda (16.87%), whereas no variation was observed between genotypes representing A. atlantica, A. longiglumis, A. insulanis, and A. sterilis. Although in our study, individual Avena species were represented by an unequal number of accessions, the level of polymorphism revealed for particular species seems not to be associated with the sample size: both species represented by the smallest number of accessions (A. longiglumis, A. atlantica, A. insulanis) as well as these represented by the highest number of genotypes (A. byzantiana and A. sterilis) can be found among species with the lowest polymorphism. Fu and Williams (2008) in their AFLP studies of 25 Avena species reported 7.3% to 62.7% of polymorphic bands for a species. Moreover, the authors noticed a positive correlation between the number of polymorphic bands and the number of accessions representing particular species.
So far, iPBS markers were used only once in genetic studies of Avena species (Nečajeva et al. 2021). The study revealed that the proportion of polymorphic bands within eighteen Latvian, one Polish, and one Norway A. fatua populations ranged from 47 to 85%, but when the number of polymorphic fragments revealed by a particular PBS primer was considered, the values were even higher and ranged from 88.9 to 100%. Application of other molecular tools in previously published genetic studies of various Avena species revealed the differentiated level of polymorphism. For example, analyses of 16 Avena species with the use of RAPD markers yielded from 62.5 to 100% polymorphic bands per primer (Sood et al. 2016), whereas even 90% to 100% of RAPD polymorphic bands per primer was reported by Drossou et al. (2004) during the study of relationships between 18 Avena species. Application of AFLP markers allowed to identify of about 42.8% of polymorphic bands during the analysis of the genetic diversity of 96 oat cultivars (Fu et al. 2003), but there are also examples in which AFLP markers can reveal up to 97.4% (Drossou et al. 2004) of polymorphic loci in analyzed Avena species. In the case of ISSR markers, the revealed polymorphism varies between 41.7% (Paczos-Grzęda 2007) and 70.9% (Paczos-Grzęda et al. 2009) in the studies of A. sativa and A. sterilis, respectively, whereas application of codominant SSR markers allowed to observe even 100% of polymorphic loci (Sood et al. 2016). Although the reported in this paper’s level of polymorphism of studied Avena collection was lower than in the case of other mentioned molecular tools, it appeared to be sufficient to distinguish analyzed Avena species and the majority of accessions. Our observations, together with the results of the study by Nečajeva et al. (2021) proved that iPBS markers should be treated as a cost-efficient and reliable source of molecular data in genetic studies of Avena species and noteworthy alternative for RAPD markers, which are said to suffer from a low reproducibility of the results (Kumari and Thakur 2014), laborious and technically demanding AFLP markers (Karaca and Ince 2017), or SSRs which are time-consuming and expensive to develop (Yang et al. 2013).
The current study showed that most of the iPBS variation was located among studied Avena species, among groups of Avena species which belong to different genome types, among groups of Avena species which share the same ploidy level, and among Avena sections proposed by Baum (1977). The groups above are given in descending order of the value of the percentage of variation identified among them. Only about one-third of the total genetic variation resided within individual species. These observations are congruent with the results described by Fu and Williams (2008) who also reported that most of the total genetic variation is partitioned among studied Avena species (59.5%) and only about 40.5% within individual species.
UPGMA clustering of 60 Avena accessions based on the Dice’s similarity coefficient generated a dendrogram in which two main clusters were clearly distinguished. Cluster I, which gathered 25 accessions, included genotypes representing all diploid Avena species, two tetraploid oats (A. abyssynica and A. barbata), and one accession representing hexaploid A. fatua. The remaining 35 Avena accessions could be found within cluster II, which gathered genotypes representing all hexaploid Avena species together with tetraploid A. abyssynica and A. insulanis and two accessions of diploid A. fatua and one accession of tetraploid A. barbata. High genetic similarity between A. sativa, A. byzantiana, A. sterilis, and A. fatua revealed here by iPBS markers is concordant which previous observations concerning these hexaploid Avena species. As mentioned above, four oat species have identical ACD genome type, and share similar morphology and similar C-banding pattern of chromosomes (Rajharthy and Thomas 1974; Badaeva et al. 2011). Available results of molecular studies (Li et al. 2000b; Fu and Williams 2008) as well as observations of free crosses between these species (Loskutov 2001) also confirm their close evolutionary relationships. High similarity between tetraploid A. insulanis (with AC genome) and four hexaploid oat species with the ACD could be also noticed. This observation is in agreement with the previous findings by Rajharthy and Thomas (1974), who stated that tetraploid AC-genome species were sources of the hexaploid oat A and C genomes. Moreover, the lack of significant differences in the banding pattern of chromosomes between A. insularis and two other tetraploid oats (A. magna and A. murphyi) confirmed the major role of AC genome teraploids in the evolution of hexaploid Avena species (Jellen and Ladizinsky 2000). When two other studied tetraploid oats A. abyssynica and A. barbata representing the AB genome group were considered, their higher genetic differentiation was observed (based on the pattern of the scattering of their accessions between cluster I and cluster II), as well as their closer relationship with the diploid oats. The close relationship between AB and A genome Avena species was reported also by Fu (2018) based on an analysis of chloroplast SNPs. Phylogenetic studies suggest that the most plausible ancestor of the A genome for A. abyssynica and A. barbata is an As-genome species; however, the second ancestor of these two species has not been identified yet, but most likely, it does not belong to the As-genome group (Loskutov and Rines 2011). According to the available literature, it could be A. damascena as it was proposed for A. agadiriana, another AB genome tetraploid (Badaeva et al. 2010). This theory may be supported by our findings where A. damasecna can be found in one subcluster within cluster I together with A. abyssynica and A. barbata. Furthermore, all diploid Avena species were gathered in one cluster, confirming their common genetic features reported previously by the means of molecular (Fu and Williams 2008) and cytological studies (Rajharthy and Thomas 1974; Yan et al. 2016). Finally, although the accessions representing a particular species or shearing the same type of genome tend to cluster together, there were some samples representing, e.g., A. nuda and A. fatua, which did not follow that rule. Scattering of these accessions between different Avena clusters may be interpreted as their higher genetic variation revealed by iPBS markers and/or not clear relationships of the particular taxon with other oats as it is in the case of A. nuda (Baum 1977). The analogical situation, i.e., clustering of some genotypes representing particular Avena species separately, with other oats, was also observed, e.g., by Li et al. (2009) by the means of consensus chloroplast SSRs and Sood et al. (2016) based on the results of RAPD and SSR markers.
Additionally, to infer about relationships between studied Avena species, the PCoA was applied based on Nei’s genetic distance values estimated between all pairs of oat species. Also in this approach, the observed pattern of genetic variation clearly corresponded to the ploidy level of the analyzed species, pointing on a different character of diploid and hexaploid Avena species which were grouped separately, and intermediate character of tetraploid oats, in which AB genome oats (A. abyssynica and A. barbata) were more similar to diploid oats, but A. insularis (AC genome) revealed close affinity to hexaploid Avena species. As in both mentioned above approaches, i.e., when individual Avena accessions were considered or the species level was analyzed, the genome type and ploidy level appeared to play the major role in the clustering process, the additional analysis was performed in which the accessions were grouped according to their genome type. Also, in this analysis, the AC genome tetraploids revealed the closest relationships with ACD genome hexaploids, whereas AB genome tetraploids were clustered with As and Ad diploids. The Al genome diploid appeared here as the most distinct. Our results confirmed previous observations of Katsiotis et al. (1997) who reported that the A and B genomes reveal close relationships and that the barbata group of teraploids (AB genome) arose from As diploids through autoteraploidization. The high affinity between As genome and AB genome tetraploid was reported also by Nikoloudakis et al. (2008). According to the authors (Nikoloudakis et al. 2008), the distinction between the A and B genomes was not possible, while the major genetic divergence between the A and C genomes was observed. C genome appeared to be approximately 15% longer than the A genome (Yan et al. 2016) most likely due to differences in repetitive DNA content (Jellen et al. 1993). Moreover, mostly asymmetrical, heterochromatic chromosomes are characteristic for Avena species carrying the C genome, while symmetrical karyotype and low content of heterochromatin are found in species with A genome (Fominaya et al. 1988).
The revealed pattern of interspecific variation of studied Avena species appeared to be concordant also with the grouping of oat species in terms of their crossability proposed by Harlan and de Wet (1971). That gene pool classification system divided the Avena species into three groups (gene pools). The primary gene pool, following the Ladizinsky and Zohary (1971), included all cultivated and wild hexaploid oat taxa that cross with each other easily. The secondary gene pool, according to Leggett and Thomas (1995), gathers AC tetraploid species A. magna, A, murphhyi, and A. insularis for which successful hybridization with hexaploid Avena species was reported, although some problems with the fertility of the hybrids could be observed. Finally, the tertiary gene pool was distinguished (Leggett and Thomas 1995), which consisted of all diploid Avena species and the tetraploid A. abysinnica, A. barbata, A. vaviloviana, and A. macrostachya. Although mentioned above, species do not readily hybridize with A. sativa, but with the application of some additional in vitro techniques (e.g., embryo rescue), successful trait introgression could be observed (Loskutov and Rines 2011).
Soluble carbohydrates content and composition
Cereals are cultivated generally for their seeds suitable for food and/or livestock feed. Cereal grains are rich in carbohydrates, with comparatively low content of protein, fats, vitamins, and minerals. In the case of the oat grains, 75–80% of their dry matter are carbohydrates (mostly polysaccharides), whereas protein content is about 11–15% (Rodehutscord et al. 2016), but there are also observations according to which protein content in wild oat species may reach 27–28% (Campbell and Frey 1972) or even 35% (Frey 1975). In addition to proteins, oat grains can be rich also in fats, as it was observed in some diploid and tetraploid species, for which the content of oil in grains may reach up to 12–13% (Welch and Leggett 1997). When the carbohydrate content in oat grains is considered, the main focus of scientists is generally focused so far on the starch and β-glucans because of their role in grain digestibility or beneficial effects on human health (e.g., Davidson et al. 1991). The starch comprises the largest fraction of the carbohydrates and shares up to 73.4% of grains’ dry weight, as it was observed in A. sativa (Beloshapka et al. 2016), whereas β-glucans content may vary from 2.2 to 11.3%, depending on the species (Welch et al. 2000). In case of soluble carbohydrates, their qualitative and quantitative composition in oat grains was less frequently studied.
The results of our study showed the usefulness of intra- and inter-specific variation in soluble carbohydrate profiles in caryopses for the characterization of the Avena species. The results of capillary gas chromatography showed that all studied Avena accessions had the same qualitative composition of soluble carbohydrate profiles, but with significant quantitative differences between individual components. Sucrose appeared to have the largest share in the total carbohydrate content, regardless of the analyzed accession or species. According to our data, sucrose constitutes on average 59.9% of all identified sugars reaching the highest values in A. atlantica (73.3%), A. byzantina, and A. nuda (69.3% and 69.2%, respectively). The highest content of sucrose among free sugars and oligosaccharides in seeds of 22 grass species (MacLeod and McCorquodale 1958) as well as different cereal grains (MacLeod and Preece 1953) was also reported. Oat with 639 mg of sucrose per 100 g of grain took the last position in this ranking after rye, barley, wheat, and maize (1857 mg, 908 mg, 836 mg, and 783 mg, respectively; MacLeod and Preece 1953), but also was found in the middle of the rank (11th out of 22) of the studied grass species for which the sucrose content ranged from 194 mg for Agropyron repens up to 10700 mg for Spartina townsendii (MacLeod and McCorquodale 1958). Glucose and fructose were also encountered in all analyzed Avena accessions but these monosaccharides comprise on average only 0.96% of total identified carbohydrates. Both papers cited above (MacLeod and Preece 1953, MacLeod and McCorquodale 1958) also reported the presence of glucose and fructose in all analyzed seeds, but always as a minor component.
Although plants generally store carbohydrates in long polysaccharide chains (e.g., starch, glucans), sucrose can also be found among storage molecules in roots, leaves, fruits, and seeds. The importance of sucrose is associated also with its central position in plant metabolism, as it is the end product of photosynthesis, the primary sugar transported in phloem and carbon skeleton for the synthesis of organic matter such as amino acids or nucleotides (Stein and Granot 2019). In seeds, sucrose is cleaved into monosaccharides, being the source of carbon skeletons and energy for developing embryo and storage tissues. During seed maturation, sucrose is also a substrate for the synthesis of other types of sucrose-derived oligosaccharides like RFOs (Keller and Pharr 1996) or fructans, present in cereals (Valluru and Van den Ende 2008). In this pathway, myo-inositol plays a significant role as the acceptor of galactose moiety in the synthesis of galactinol, a major donor of galactosyl residues for the synthesis of its higher homologues, as stachyose and verbascose (and less abundant ajugose), found in elevated amounts in legumes (Obendorf and Górecki 2012). In cereal grains, raffinose dominates among RFOs, whereas stachyose is present at a very low level (Henry and Saini 1989; Lahuta and Goszczyńska 2010). In our study, raffinose and stachyose were identified in caryopses of all studied Avena accessions, with higher raffinose content (raffinose:stachyose ratio ranged from 1.09 for A. insulanis up to 1.94 for A. strigosa). The total RFOs level was lower only from sucrose and varied from 21.6% (A. abyssynica) up to 49.9% (A. insulanis) of total carbohydrates. The content of raffinose, stachyose, and sucrose were not correlated; however, the percentage content of sucrose and total RFOs in total soluble carbohydrates revealed a significant negative correlation (r = −0.976, p < 0.001). The same phenomena was described for barley grains, where the incorporation of sucrose into RFOs reduced the final sucrose pool (Karner et al. 2004).
The central position of galactinol in the synthesis of RFOs was confirmed in our study, as the galactinol content appeared to be positively correlated with the raffinose (r = 0.669, p < 0.05) and stachyose (r = 0.579, p < 0.05) level. This is concordant with the observations of Handley et al. (1983), who suggested that the concentration of galactinol is the key factor for the RFOs content. Interestingly, no significant correlation was observed between myo-inositol and RFOs contents as it was expected based on the reports in which reduction of myo-inositol level decreased galactinol and raffinose in potato (Keller et al. 1998) and soybean (Hitz et al. 2002) or increased level of galactinol was observed in response to elevated myo-inositol (Karner et al. 2004). Nevertheless, the reported here low level of myo-inositol did not appear as a factor which limited the galactinol and RFOs content in grains of studied Avena accessions. The very low level of myo-inositol (in most oat accessions below 0.2 mg g−1 DW) could be explained by its utilization for the synthesis of phytic acid (Angel et al. 2002).
There are many factors which affect the amount of carbohydrates stored in the seeds, i.e., the availability of substrates for the synthesis of particular carbohydrate and activities of enzymes catalyzing its synthesis and degradation. If we accept that the enzyme balance undergoes genetic control, then the amounts of individual carbohydrates depend on the genetic composition of plants (MacLeod and McCorquodale 1958). As a consequence, the differentiation of carbohydrate profiles can be observed between species, especially when they represent distant lineages, as it was observed by MacLeod and McCorquodale (1958) in their studies of 22 Gramineae species or among legume species (Obendorf and Górecki 2012). In the case of the current study, high quantitative differentiation in carbohydrate profiles was found, both within studied species as well as between them; however, no species-specific carbohydrate fractions were found. But even then, the results of PCA analysis based on obtained carbohydrate data showed an interesting pattern of interspecific variation, in which diploid Avena species tend to form a central group, surrounded by the other oats, with hexaploid species scattered generally in the external areas of the 2D PCA-plot, whereas tetraploid species took an intermediate position. However, we are aware of the fact that identified carbohydrates represent no more than 2% of the dry weight of the analyzed grains, so analysis of other components may be also suggested in case of detailed characteristic and/or discriminatory analysis of species representing genus Avena.
Conclusions
Here, we reported the results of the first use of the retrotransposon-based iPBS markers for assessment of the rate of genome divergence within and between species representing genus Avena, combined with the analysis of soluble carbohydrate profiles. Both methods applied in our study revealed their discriminatory power for studied Avena accessions and allowed to perform their comparison. Molecular data obtained with the use of retrotransposon-based iPBS markers showed that the TEs activity accompanied the Avena genome’s natural evolution and domestication. Polymorphism revealed by iPBS markers confirmed previous observations according to which oat species can be differentiated based on their ploidy level, i.e., into a group of diploid and a group of hexaploid species which share the lowest similarity, and a group of tetraploid species with rather intermediate character. iPBS data confirmed also evolutionary relationships between Avena genome types revealing high similarity between hexaploid ACD genome and tetraploid AC genome, and close affinity of tetraploid AB genome with diploid A genomes.
Analysis of soluble carbohydrate profiles showed that studied Avena accessions share the same quantitative composition, but significant differences in the quantity of particular sugars among studied samples were observed. Application of capillary gas chromatography allowed us to identify a group of ten soluble carbohydrates among which sucrose appeared as the most abundant. Based on the carbohydrate profiles, the relations between studied Avena species were studied. The results of PCA analysis revealed an interesting pattern of interspecific variation, convergent to that revealed by iPBS markers. Both molecular and biochemical data presented in our paper not only complement our knowledge about the diversity found within and among Avena species, but they may be also used as an important argument in the discussion on the evolution of the whole genus. Such information may also become valuable data useful for breeders who need detailed characteristics of breeding material for the development of successful and efficient breeding programs.
References
Alicchio R, Aranci L, Conte L (1995) Restriction fragment length polymorphism based phylogenetic analysis of Avena L. Genome 38:1279–1284. https://doi.org/10.1139/g95-168
Almojil D, Bourgeois Y, Falis M, Hariyani I, Wilcox J, Boissinot S (2021) The structural, functional and evolutionary impact of transposable elements in eukaryotes. Genes 12:918. https://doi.org/10.3390/genes12060918
Andersson A, Börjesdotter D (2011) Effects of environment and variety on content and molecular weight of β-glucan in oats. J Cereal Sci 54:122–128. https://doi.org/10.1016/j.jcs.2011.03.003
Andon MB, Anderson JW (2008) State of the art reviews: the oatmeal-cholesterol connection: 10 years later. Am J Lifestyle Med 2:51–57. https://doi.org/10.1177/1559827607309130
Androsiuk P, Chwedorzewska KJ, Szandar K, Giełwanowska I (2015) Genetic variability of Colobanthus quitensis from King George Island (Antarctica). Pol Polar Res 36:281–295. https://doi.org/10.1515/popore-2015-0017
Angel R, Tamim NM, Applegate TJ, Dhandu AS, Ellestad LE (2002) Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. J Appl Poult Res 11:471–480. https://doi.org/10.1093/japr/11.4.471
Ates D, Altinok HH, Ozkuru E, Ferik F, Erdogmus S, Can C, Tanyolac MB (2019) Population structure and linkage disequilibrium in a large collection of Fusarium oxysporum strains analysed through iPBS markers. J Phytopathol 167:576–590. https://doi.org/10.1111/jph.12848
Badaeva ED, Shelukhina OY, Gorunova SV, Loskutov IG, Pukhalskiy VA (2010) Phylogenetic relationships of tetraploid AB-genome Avena species evaluated by means of cytogenetic (C-banding and FISH) and RAPD analyses. Aust J Bot 742307. https://doi.org/10.1155/2010/742307
Badaeva ED, Shelukhina OY, Dedkova OS, Loskutov IG, Pukhalskiy VA (2011) Comparative cytogenetic analysis of hexaploid Avena L. species. Russ J Genet 47:691–702. https://doi.org/10.1134/S1022795411060068
Baum BR (1977) Oats: wild and cultivated: a monograph of the genus Avena L. (Poaceae). Minister of Supply and Services: Ottawa, Ontario, Canada.
Beer SC, Goffreda J, Phillips TD, Murphy JP, Sorrels ME (1993) Assessment of genetic variation in Avena sterilis using morphological traits, isozymes and RFLPs. Crop Sci 33:1386–1393. https://doi.org/10.2135/cropsci1993.0011183X003300060051x
Beloshapka AN, Buff PR, Fahey GC, Swanson KS (2016) Compositional analysis of whole grains, processed grains, grain co-products, and other carbohydrate sources with applicability to pet animal nutrition. Foods 5:23. https://doi.org/10.3390/foods5020023
Bhouri AM, Flamini G, Chraief I, Hammami M (2016) Aromatic compounds and soluble carbohydrate profiles of different varieties of Tunisian raisin (Vitis Vinifera L.). Int J Food Prop 19:339–350. https://doi.org/10.1080/10942912.2015.1027920
Boczkowska M, Tarczyk E (2013) Genetic diversity among Polish landraces of common oat (Avena sativa L.). Genet Resour Crop Evol 60:2157–2169. https://doi.org/10.1007/s10722-013-9984-1
Bonchev GN (2016) Useful parasites: the evolutionary biology and biotechnology applications of transposable elements. J Genet 95:1039–1052. https://doi.org/10.1007/s12041-016-0702-6
Bonchev GN, Vassilevska-Ivanova R (2020) Fingerprinting the genetic variation and intergeneric hybrid dynamics in the family Asteraceae (genera Helianthus, Echinaceae, Tagetes and Verbesina) using iPBS markers. Biologia 75:457–464. https://doi.org/10.2478/s11756-019-00363-3
Bonchev G, Dušinský R, Hauptvogel P, Gaplovská-Kyselá K, Švec M (2019) On the diversity and origin of the barley complex agriocrithon inferred by iPBS transposon markers. Genet Resour Crop Evol 66:1573–1586. https://doi.org/10.1007/s10722-019-00814-5
Bourque G, Burns KH, Gehring M, Gorbunova V, Seluanov A, Hammell M, Imbeault M, Izsvák Z, Levin HL, Macfarlan TS, Mager DL, Feschotte C (2018) Ten things you should know about transposable elements. Genome Biol 19:199. https://doi.org/10.1186/s13059-018-1577-z
Budny M, Zalewski K, Lahuta LB, Stolarski MJ, Stryiński R, Okorski A (2021) Could the content of soluble carbohydrates in the young shoots of selected willow cultivars be a determinant of the plants’ attractiveness to cervids (Cervidae, Mammalia)? Agriculture 11:67. https://doi.org/10.3390/agriculture11010067
Campbell AR, Frey KJ (1972) Association between groat-protein percentage and certain plant and seed traits in interspecific oat crosses. Euphytica 21:352–362. https://doi.org/10.1007/BF00036776
Constandinou S, Nikoloudakis N, Kyratzis AC, Katsiotis A (2018) Genetic diversity of Avena ventricosa populations along an ecogeographical transect in Cyprus is correlated to environmental variables. PLoS One 13:e0193885. https://doi.org/10.1371/journal.pone.0193885
Craig IL, Murray BE, Rajhathy T (1972) Leaf esterase isozymes in Avena and their relationship to the genomes. Can J Genet Cytol 14:581–589. https://doi.org/10.1139/g72-071
Daou C, Zhang H (2012) Oat Beta-Glucan: its role in health promotion and prevention of diseases. Compr Rev Food Sci F 11:355–365. https://doi.org/10.1111/j.1541-4337.2012.00189.x
Davidson MH, Dugan LD, Buras JH, Bova J, Story K, Dreenan KB (1991) The hypocholesterolemic effects of oat b-glucan in oat meal and oat bran: A dose controlled study. J Am Med Assoc 265:1833–1839. https://doi.org/10.1001/jama.1991.03460140061027
Demirel U, Tındaş İ, Yavuz C, Baloch F, Çalışkan M (2018) Assessing genetic diversity of potato genotypes using inter-PBS retrotransposon marker system. Plant Genet Resour 16:137–145. https://doi.org/10.1017/S1479262117000041
Dice L (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302. https://doi.org/10.2307/1932409
Drossou A, Katsiotis A, Leggett JM, Loukas M, Tsakas S (2004) Genome and species relationships in genus Avena based on RAPD and AFLP molecular markers. Theor Appl Genet 109:48–54. https://doi.org/10.1007/s00122-004-1615-y
Erper I, Ozer G, Kalendar R, Avci S, Yildirim E, Alkan M, Turkkan M (2021) Genetic diversity and pathogenicity of Rhizoctonia spp. isolates associated with red cabbage in Samsun (Turkey). J Fungi (Basel) 7:234. https://doi.org/10.3390/jof7030234
Excoffier L, Smouse P, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction sites. Genetics 131:479–491. https://doi.org/10.1093/genetics/131.2.479
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinforma 1:47–50. https://doi.org/10.1177/117693430500100003
Fardet A (2010) New hypotheses for the health-protective mechanisms of wholegrain cereals: what is beyond fibre? Nutr Res Rev 23:65–134. https://doi.org/10.1017/s0954422410000041
Feschotte C, Pritham EJ (2007) DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet 41:331–368. https://doi.org/10.1146/annurev.genet.40.110405.090448
Feschotte C, Jiang N, Wessler SR (2002) Plant transposable elements: where genetics meets genomics. Nat Rev Genet 3:329–341. https://doi.org/10.1038/nrg793
Flavell A, Knox MR, Pearce SR, Ellis THN (1998) Retrotransposon-based insertion polymorphisms (RBIP) for high throughput marker analysis. Plant J 16:643–665. https://doi.org/10.1046/j.1365-313x.1998.00334.x
Fominaya A, Vega P, Ferrer E (1988) Giemsa C-banded karyotypes of Avena species. Genome 30:627–632. https://doi.org/10.1139/g88-106
Frey KJ (1975) Inheritability of groat-protein percentage of hexaploid oats. Crop Sci 15:277–279. https://doi.org/10.2135/cropsci1975.0011183X001500020042x
Frey KJ (1991) Genetic resources of oats. In: Shands HL, Wiesner LE (ed) Use of plant introductions in cultivar development. CSSA Special Publication, Part 1, No 17, Madison, WI, USA, pp 15–24
Frost LS, Leplae R, Summers AO, Toussaint A (2005) Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol 3:722–732. https://doi.org/10.1038/nrmicro1235
Fu YB (2018) Oat evolution revealed in the maternal lineages of 25 Avena species. Sci Rep 8:4252. https://doi.org/10.1038/s41598-018-22478-4
Fu YB, Williams DJ (2008) AFLP variation in 25 Avena species. Theor Appl Genet 117:333–342. https://doi.org/10.1007/s00122-008-0778-3
Fu YB, Peterson GW, Scoles G, Rossnagel B, Schoen DJ, Richards KW (2003) Allelic diversity changes in 96 Canadian oat cultivars released from 1886 to 2001. Crop Sci 43:1989–1995. https://doi.org/10.2135/cropsci2003.1989
Gordon TC, Jin Y, Tinker NA, Bekele WA, Gale S, Bockelman H, Bonman M (2022) Comparative sequencing and SNP marker validation for oat stem rust resistance gene Pg6 in a diverse collection of Avena accessions. Theor Appl Genet 135:1307–1318. https://doi.org/10.1007/s00122-022-04032-z
Govindaraj M, Vetriventhan M, Srinivasan M (2015) Importance of genetic diversity assessment in crop plants and its recent advances: an overview of its analytical perspectives. Genet Res Int 2015:431487. https://doi.org/10.1155/2015/431487
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol Electron 4:9
Handley LW, Pharr DM, McFeeters RF (1983) Relationship between galactinol synthase activity and sugar composition of leaves and seeds of several crop species. J Am Soc Hortic Sci 108:600–605. https://doi.org/10.21273/JASHS.108.4.600
Harlan JR, de Wet JMJ (1971) Toward a rational classification of cultivated plants. Taxon 20:509–517. https://doi.org/10.2307/1218252
Henry RJ, Saini HS (1989) Characterization of cereal sugars and oligosaccharides. Cereal Chem 66:362–365
Heun M, Murphy JP, Phillips TD (1994) A comparison of RAPD and isozyme analyses for determining the genetic relationships among Avena sterilis L. accessions. Theor Appl Genet 87:689–696. https://doi.org/10.1007/bf00222894
Hitz WD, Carlson TJ, Kerr PS, Sebastian SA (2002) Biochemical and molecular characterization of a mutation that confers a decreased raffinosaccharide and phytic acid phenotype on soybean seeds. Plant Physiol 128:650–660. https://doi.org/10.1104/pp.010585
Hofmann LA (1995) World production and use of oats. In: Welch RW (ed) The oat crop: Production and utilization, Chapman and Hall, London, UK, pp 34–61
de la Hoz PS, Fominaya A (1989) Studies of isozymes in oat species. Theoret Appl Genetics 77:735–741. https://doi.org/10.1007/BF00261252
Ibrahim N, Taleb M, Heiss AG, Kropf M, Farag MA (2021) GC-MS based metabolites profiling of nutrients and anti-nutrients in 10 Lathyrus seed genotypes: a prospect for phyto-equivalency and chemotaxonomy. Food Biosci 42:101183. https://doi.org/10.1016/j.fbio.2021.101183
Jellen EN, Ladizinsky G (2000) Giemsa C_banding in Avena insularis Ladizinsky. Genet Resour Crop Evol 47:227–230. https://doi.org/10.1023/A:1008769105071
Jellen EN, Phillips RL, Rines HW (1993) C-banded karyotypes and polymorphisms in hexaploid oat accessions (Avena spp.) using Wright’s stain. Genome 36:1129–1137. https://doi.org/10.1139/g93-151
Jenkins AL, Jenkins DJA, Zdravkovic U, Wursch P, Vuksan V (2002) Depression of the glycemic index by high levels of beta-glucan fiber in two functional foods tested in type 2 diabetes. Eur J Clin Nutr 56:622–628. https://doi.org/10.1038/sj.ejcn.1601367
Kalendar R, Grob T, Regina M, Suoniemi A, Schulman A (1999) IRAP and REMAP: two retrotransposon-based DNA fingerprinting techniques. Theor Appl Genet 98:704–711. https://doi.org/10.1007/s001220051124
Kalendar R, Antonius K, Smýkal P, Schulman AH (2010) iPBS: a universal method for DNA fingerprinting and retrotransposon isolation. Theor Appl Genet 121:1419–1430. https://doi.org/10.1007/s00122-010-1398-2
Kalendar R, Amenov A, Daniyarov A (2018) Use of retrotransposon-derived genetic markers to analyse genomic variability in plants. Funct Plant Biol 46:15–29. https://doi.org/10.1071/FP18098
Karaca M, Ince AG (2017) Molecular markers in Salvia L.: past, present and future. In Georgiev V, Pavlov A (ed) Salvia Biotechnology, Springer International Publishing AG: Cham, Switzerland, pp.291-398. https://doi.org/10.1007/978-3-319-73900-7_9
Karagoz H, Cakmakci R, Hosseinpour A, Ozkan G, Haliloglu K (2020) Analysis of genetic variation and population structure among of oregano (Origanum acutidens L.) accessions revealed by agro-morphological traits, oil constituents and retrotransposon-based inter-primer binding sites (iPBS) markers. Genet Resour Crop Evol 67:1367–1384. https://doi.org/10.1007/s10722-020-00887-7
Karner U, Peterbauer T, Raboy V, Jones DA, Hedley CL, Richter A (2004) myo-inositol and sucrose concentrations affect the accumulation of raffinose family oligosaccharides in seeds. J Exp Bot 55:1981–1987. https://doi.org/10.1093/jxb/erh216
Katsiotis A, Schmidt T, Heslop-Harrison JS (1996) Chromosomal and genomic organization of Ty1-copia-like retrotransposon sequences in the genus Avena. Genome 39:410–417. https://doi.org/10.1139/g96-052
Katsiotis A, Hagidimitriou M, Heslop-Harrison JS (1997) The close relationship between the A and B genomes in Avena L. (Poaceae) determined by molecular cytogenetic analysis of total genomic, tandemly and dispersed repetitive DNA sequences. Ann Bot-London 79:103–109. https://doi.org/10.1006/anbo.1996.0312
Kaur S, Bhardwaj RD, Kapoor R, Grewal SK (2019) Biochemical characterization of oat (Avena sativa L.) genotypes with high nutritional potential. LWT – Food. Sci Technol 110:32–39. https://doi.org/10.1016/j.lwt.2019.04.063
Keller F, Pharr DM (1996) Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosaccharides. In: Zamski E, Schaffer AA (ed) Photoassimilate distribution in plants and crops: Source–Sink Relationships,1st ed., Marcel Dekker Inc. New York, USA, pp. 157–183.
Keller R, Brearley CA, Trethewey RN, Müller-Röber B (1998) Reduced inositol content and altered morphology in transgenic potato plants inhibited for 1D-myo-inositol 3-phosphate synthase. Plant J 16:403–410. https://doi.org/10.1046/j.1365-313x.1998.00309.x
Khapilina O, Raiser O, Danilova A, Shevtsov V, Turzhanova A, Kalendar R (2021) DNA profiling and assessment of genetic diversity of relict species Allium altaicum Pall. on the territory of Altai. PeerJ 9:e10674. https://doi.org/10.7717/peerj.10674
Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet 33:479–532. https://doi.org/10.1146/annurev.genet.33.1.479
Kumari N, Thakur SK (2014) Randomly amplified polymorphic DNA – a brief review. Am J Anim Vet Sci 9:6–13. https://doi.org/10.3844/ajavssp.2014.6.13
Kunze R, Saedler H, Lönnig WE (1997) Plant transposable elements. Adv Bot Res 27:331–470
Ladizinsky G, Zohary D (1971) Notes on species delimination species relationships and poliploidy in Avena L. Euphytica 20:380–395. https://doi.org/10.1007/BF00035663
Lahuta LB (2006) Biosynthesis of raffinose family oligosaccharides and galactosyl pinitols in developing and maturing seeds of winter vetch (Vicia vlllosa Roth.). Acta Soc Bot Pol 75:219–227. https://doi.org/10.5586/asbp.2006.026
Lahuta LB, Goszczyńska J (2010) Cyclitols in maturing grains of wheat, triticale and barley. Acta Soc Bot Pol 79:181–187. https://doi.org/10.5586/asbp.2010.023
Lahuta LB, Ciak M, Rybiński W, Bocianowski J, Börner A (2018) Diversity of the composition and content of soluble carbohydrates in seeds of the genus Vicia (Leguminosae). Genet Resour Crop Evol 65:541–554. https://doi.org/10.1007/s10722-017-0552-y
Lahuta LB, Rybiński W, Bocianowski J, Nowosad K, Börner A (2020) Raffinose family oligosaccharides in seeds of common vetch (Vicia sativa L. ssp. sativa). Legum Res 43:512–517. https://doi.org/10.18805/LR-491
Leggett JM (1996) Using and conserving Avena genetic resources. In: Scoles GJ, Rossnagel BG (eds) Proceedings of the Vth international oat conference and VIIth international barley genetic symposium. University of Saskatchewan, Saskatoon, Canada, pp 128–132
Leggett JM, Thomas H (1995) Oat evolution and cytogenetics. In: Welch RW (ed) The oat crop production and utilization. Chapman and Hall, London, UK, pp 120–149
Leonard KJ, Anikster Y, Manisterski J (2004) Patterns of virulence in natural populations of Puccinia coronate on wild oat in Israel and in agricultural populations on cultivated oat in the United States. Phytopathology 94:505–514. https://doi.org/10.1094/phyto.2004.94.5.505
Levin HL, Moran JV (2011) Dynamic interactions between transposable elements and their hosts. Nat Rev Genet 12:615–627. https://doi.org/10.1038/nrg3030
Li CD, Rossnagel BG, Scoles GJ (2000a) The development of oat microsatellite markers and their use in identifying relationships among Avena species and oat cultivars. Theor Appl Genet 101:1259–1268. https://doi.org/10.1007/s001220051605
Li CD, Rossnagel BG, Scoles GJ (2000b) Tracing the phylogeny of the hexaploid oat Avena sativa with satellite DNAs. Crop Sci 40:1755–1763. https://doi.org/10.2135/cropsci2000.4061755x
Li WT, Peng YY, Wei YM, Baum BR, Zheng YL (2009) Relationships among Avena species as revealed by consensus chloroplast simple sequence repeat (ccSSR) markers. Genet Resour Crop Evol 56:465–480. https://doi.org/10.1007/s10722-008-9379-x
Linares C, Ferrer E, Fominaya A (1998) Discrimination of closely related A and D genomes of the hexaploidy oat Avena sativa. L. Proc Natl Acad Sci U S A 95:12450–12455. https://doi.org/10.1073/pnas.95.21.12450
Linares C, Serna A, Fominaya A (1999) Chromosomal organization of a sequence related to LTR-like elements of Ty1-copia retrotransposons in Avena species. Genome 42:706–713. https://doi.org/10.1139/g99-007
Loskutov IG (2001) Interspecific crosses in the genus Avena L. Russ J Genet 37:467–475. https://doi.org/10.1023/A:1016697812009
Loskutov IG, Rines HW (2011) Chapter 3: Avena. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, cereals, Springer-Verlag: Berlin Heidelberg, Germany. https://doi.org/10.1007/978-3-642-14228-4_3
Loskutov IG, Gnutikov AA, Blinova EV, Rodionov AV (2021) The origin and resource potential of wild and cultivated species of the genus of oats (Avena L.). Russ J Genet 57:642–661. https://doi.org/10.1134/S1022795421060065
MacLeod AM, McCorquodale H (1958) Water-soluble carbohydrates of seeds of Gramineae. New Phytol 57:168–182. https://doi.org/10.1111/j.1469-8137.1958.tb05303.x
MacLeod AM, Preece IA (1953) Studies on the free sugars of barley grain. V. Comparison of sugars and fructosans with those of other cereals. J Inst Brew 60:46–55. https://doi.org/10.1002/j.2050-0416.1954.tb02747.x
Mal B (1987) Wild genetic resource potential for forage oat improvement (Abstr). In: 1st Symposium on crop improvement, 23–27 Feb 1987, India, pp 5–6.
Mancilla-Margalli NA, López MG (2006) Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion Species. J Agric Food Chem 54:7832–7839. https://doi.org/10.1021/jf060354v
Marshall DR, Allard RW (1970) Isozyme polymorphisms in natural populations of Avena fatua and A. barbata. Heredity 25:373–382. https://doi.org/10.1038/hdy.1970.38
Marshall HG, Shaner GE (1992) Genetics and inheritance in oat. In: Sorrels ME (ed) Oat science and technology, Agronomy N13, USA, pp 509–571.
Maughan PJ, Lee R, Walstead R, Vickerstaff RJ, Fogarty MC, Brouwer CR, Reid RR, Jay JJ, Bekele WA, Jackson EW, Tinker NA, Langdon T, Schlueter JA, Jellen EN (2019) Genomic insights from the first chromosome-scale assemblies of oat (Avena spp.) diploid species. BMC Biol 17:92. https://doi.org/10.1186/s12915-019-0712-y
McClintock B (1984) The significance of responses of the genome to challenge. Science 226:792–801. https://doi.org/10.1126/science.15739260
Mehmood A, Luo S, Ahmad NM, Dong C, Mahmood T, Sajjad Y, Jaskani MJ, Sharp P (2016) Molecular variability and phylogenetic relationships of guava (Psidium guajava L.) cultivars using inter-primer binding site (iPBS) and microsatellite (SSR) markers. Genet Resour Crop Evol 63:1345–1361. https://doi.org/10.1007/s10722-015-0322-7
Milarska SE, Androsiuk P, Bednarek PT, Larson K, Giełwanowska I (2022) Genetic variation of Cerastium alpinum L. from Babia Góra, a critically endangered species in Poland. J Appl Genetics published on line 02 November 2022. https://doi.org/10.1007/s13353-022-00731-x
Miller SS, Wood PJ, Pietrzak LN, Fulcher RG (1993) Mixed linkage b-glucan, protein content and kernel weigh in Avena species. Cereal Chem 70:231–233
Moore-Colyer R (1995) Oats and oat production in history and pre-history. In: Welch R (ed) The oat crop: Production and utilization, Chapman and Hall: London, UK, pp 1–33.
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326. https://doi.org/10.1093/nar/8.19.4321
Naeem H, Awan FS, Dracatos PM, Sajid MW, Saleem S, Yousafi Q, Khan MS, Mehmood A, Zulfigar B (2021) Population structure and phylogenetic relationship of Peach [Prunus persica (L.) Batsch] and Nectarine [Prunus persica var. nucipersica (L.) C.K. Schneid.] based on retrotransposon markers. Genet Resour Crop Evol 68:3011–3023. https://doi.org/10.1007/s10722-021-01172-x
Ņečajeva J, Bleidere M, Jansone Z, Gailīte A, Ruņģis D (2021) Variability of seed germination and dormancy characteristics and genetic analysis of Latvian Avena fatua populations. Plants 10:235. https://doi.org/10.3390/plants10020235
Nei M (1972) Genetic distance between populations. Am Nat 106:283–392. https://doi.org/10.1086/282771
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590. https://doi.org/10.1093/GENETICS%2F89.3.583
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York, NY, USA. https://doi.org/10.7312/nei-92038
Nikoloudakis N, Skaracis G, Katsiotis A (2008) Evolutionary insights inferred by molecular analysis of the ITS1-5.8SITS2 and IGS Avena sp. sequences. Mol Phylogenet Evol 46:102–115. https://doi.org/10.1016/j.ympev.2007.10.007
Nikoloudakis N, Bladenopoulos K, Katsiotis A (2016) Structural patterns and genetic diversity among oat (Avena) landraces assessed by microsatellite markers and morphological analysis. Genet Resour Crop Evol 63:801–811. https://doi.org/10.1007/s10722-015-0284-9
Nocelli E, Giovannini T, Bioni M, Alicchio R (1999) RFLP- and RAPD-based genetic relationships of seven diploid species of Avena with the A genome. Genome 42:950–959. https://doi.org/10.1139/g99-029
O’Donoughue LS, Wang Z, Roder M, Kneen B, Leggett JM, Sorrells ME, Tanksley SD (1992) An RFLP-based linkage map of oats based on a cross between two diploid taxa (Avena atlantica _ A. hirtula). Genome 35:765–771. https://doi.org/10.1139/g92-117
Obendorf R, Górecki R (2012) Soluble carbohydrates in legume seeds. Seed Sci Res 22:219–242. https://doi.org/10.1017/S0960258512000104
Ociepa T (2019) The oat gene pools—review about the use of wild species in improving cultivated oat. J Cent Eur Agric 20:251–261. https://doi.org/10.5513/JCEA01/20.1.2044
Okoń S, Paczos-Grzęda E, Ociepa T, Koroluk A, Sowa S, Kowalczyk K, Chrząstek M (2016) Avena sterilis L. genotypes as a potential source of resistance to oat powdery mildew. Plant Dis 100:2145–2151. https://doi.org/10.1094/PDIS-11-15-1365-RE
Okoń S, Ociepa T, Paczos-Grzęda E, Ladizinsky G (2018) Evaluation of resistance to Blumeria graminis (DC.) f. sp. avenae, in Avena murphyi and A. magna genotypes. Crop Prot 106:177–181. https://doi.org/10.1016/j.cropro.2017.12.025
Paczos-Grzęda E (2007) Wykorzystanie metod ISSR i RAPD oraz analizy rodowodów do oceny podobieństwa międzyodmianowego Avena sativa. Zesz Probl Postep Nauk Rol 517:547–558
Paczos-Grzęda E, Bednarek PT (2014) Comparative analysis of hexaploid Avena species using REMAP and ISSR methods. Turk J Bot 38:1103–1111. https://doi.org/10.3906/bot-1403-10
Paczos-Grzęda E, Chrzastek M, Okon S, Gradzielewska A, Miazga D (2009) Zastosowanie markerów ISSR do analizy wewnątrzgatunkowego podobieństwa genetycznego Avena sterilis L. Biul Inst Hod Rośl 252:215–223
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. https://doi.org/10.1111/j.1471-8286.2005.01155.x
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Peterson DM (2001) Oat antioxidants. J Cereal Sci 33:115–129. https://doi.org/10.1006/jcrs.2000.0349
Piotrowicz-Cieslak AI (2005) Composition of seed soluble carbohydrates and ultrastructural diversity of testa in lupins from the mediterranean region. Acta Soc Bot Pol 74:281–286. https://doi.org/10.5586/asbp.2005.036
Polok K (2007) Molecular evolution of the genus Lolium L. Studio Poligrafii Komputerowej SQL: Olsztyn, Poland.
Portyanko VA, Hoffman DL, Lee M, Holland JB (2001) A linkage map of hexaploid oat based on grass anchor DNA clones and its relationship to other oat maps. Genome 44:249–265. https://doi.org/10.1139/g01-003
Rajharthy T, Thomas H (1974) Cytogenetics of Oats (Avena L.). Misc Publ Genet Soc Can 2:1–90
Rezai A (1977) Variation for some agronomic traits in the world collection of wild oats (Avena sterilis L.). Diss Abstr Int 38.11.5129B.
Rines HW, Molnar SJ, Tinker NA, Phillips RL (2006) Oat. In: Kole C (ed) Genome mapping and molecular breeding in plants, Cereals and millets, vol 1. Springer, New York, USA, pp 211–242
Rodehutscord M, Rückert C, Maurer HP, Schenkel H, Schipprack W, Bach Knudsen KE, Schollenberger M, Laux M, Eklund M, Siegert W, Mosenthin R (2016) Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch Anim Nutr 70:87–107. https://doi.org/10.1080/1745039X.2015.1133111
Roldan-Ruiz I, Dendauw J, Van Bockstaele E, Depicker A, De Loose M (2000) AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol Breed 6:125–134. https://doi.org/10.1023/A:1009680614564
Sharma P, Tiwari S, Tripathi N, Mehta AK (2016) Polymorphism analysis in advanced mutant population of oat (Avena sativa L.) using ISSR markers. Physiol Mol Biol Plants 22:115–120. https://doi.org/10.1007/s12298-015-0333-z
Sood VK, Rana I, Hussain W, Chaudhary HK (2016) Genetic diversity of genus Avena from North Western-Himalayas using molecular markers. Proc Natl Acad Sci India Sect B Biol Sci 86:151–158. https://doi.org/10.1007/s40011-014-0427-3
Stein O, Granot D (2019) An overview of sucrose synthases in plants. Front Plant Sci 10:95. https://doi.org/10.3389/fpls.2019.00095
Tajima F (1983) Evolutionary relationship of DNA sequences in finite populations. Genetics 105:437–460. https://doi.org/10.1093/genetics/105.2.437
Thomas H (1992) Cytogenetics of Avena. In: Marshall HG, Sorrells ME (ed) Oat science and technology. Monograph 33, Agronomy Series, ASA and CSSA, Madison, Wis., USA, pp 473-507.
Tomás D, Rodrigues J, Varela A, Veloso MM, Viegas W, Silva M (2016) Use of repetitive sequences for molecular and cytogenetic characterization of Avena species from Portugal. Int J Mol Sci 17:203. https://doi.org/10.3390/ijms17020203
Tomaszewska P, Schwarzacher T, Heslop-Harrison JSP (2022) Oat chromosome and genome evolution defined by widespread terminal intergenomic translocations in polyploids. Front Plant Sci 13:1026364. https://doi.org/10.3389/fpls.2022.1026364
Trofimovskaya AY, Pasynkov VI, Rodionova NA, Soldatov VN (1976) Genetic potential of section true oat of genus Avena and it importance for breeding. Works Appl Bot Genet Plant Breed 58:83–109
Turzhanova A, Khapilina ON, Tumenbayeva A, Shevtsov V, Raiser O, Kalendar R (2020) Genetic diversity of Alternaria species associated with black point in wheat grains. PeerJ 8:e9097. https://doi.org/10.7717/peerj.9097
Valluru R, Van den Ende W (2008) Plant fructans in stress environments: emerging concepts and future prospects. J Exp Bot 59:2905–2916. https://doi.org/10.1093/jxb/ern164
Waugh R, McLean K, Flavell AJ, Pearce SR, Kumar A, Thomas BBT, Powell W (1997) Genetic distribution of Bare-1-like retrotransposable elements in the barley genome revealed by sequence-specific amplification polymorphisms (S-SAP). Mol Gen Genet 253:687–694. https://doi.org/10.1007/s004380050372
Welch RW, Leggett JM (1997) Nitrogen content, oil content and oil composition of oat cultivars (A. sativa) and wild Avena species in relation to nitrogen fertility, yield and partitioning of assimilates. J Cereal Sci 26:105–120. https://doi.org/10.1006/jcrs.1996.0109
Welch RW, Brown JCW, Leggett JM (2000) Interspecific and intraspecific variation in grain and groat characteristics of wild oat (Avena) species: very high groat (1-3), (1-4)-β-D-glucan in an Avena atlantica genotype. J Cereal Sci 31:273–279. https://doi.org/10.1006/jcrs.2000.0301
Wessler SR (2006) Transposable elements and the evolution of eukaryotic genomes. Proc Natl Acad Sci U S A 103:17600–17601. https://doi.org/10.1073/pnas.0607612103
Yan H, Martin SL, Bekele WA, Latta RG, Diederichsen A, Peng Y, Tinker NA (2016) Genome size variation in the genus Avena. Genome 59:209–220. https://doi.org/10.1139/gen-2015-0132
Yang W, Kang X, Yang Q, Lin Y, Fang M (2013) Review on the development of genotyping methods for assessing farm animal diversity. J Animal Sci Biotechnol 4:2. https://doi.org/10.1186/2049-1891-4-2
Yarnell E, Abascal K (2001a) Botanical remedies for nicotine addiction. Altern Complement Ther 7:337–340. https://doi.org/10.1089/10762800152709679
Yarnell E, Abascal K (2001b) Botanical treatments for depression. Altern Complement Ther 7:138–143. https://doi.org/10.1089/107628001300303655
Yu GX, Wise RP (2000) An anchored AFLP- and retrotransposon-based map of diploid Avena. Genome 43:736–749. https://doi.org/10.1139/g00-037
Acknowledgements
Preliminary report on this study was presented in the form of poster at the scientific conference: IV Ogólnopolska Konferencja “Genetyka i genomika w doskonaleniu roślin uprawnych. Od rośliny modelowej do nowej odmiany,” 05-07.11.2019 Poznań, Poland (Milarska S, Androsiuk P, Dulska J, Kellmann-Sopyła W, Szablińska J, Lahuta LB, Giełwanowska I. Zmienność genetyczna oraz profile cukrowców rozpouszczalnych ziarniaków wybranych gatunków z rodzaju Avena). Abstracts of lectures and posters presented during the conference are available (in Polish) at https://docplayer.pl/168067607-Genetyka-i-genomika-od-rosliny-modelowej-do-nowej-odmiany-w-doskonaleniu-roslin-uprawnych-iv-ogolnopolska-konferencja-poznan-5-7-listopada-2019.html.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.A.; methodology, P.A and L.B.L.; validation, P.A and L.B.L; formal analysis, P.A.; investigation, S.M., J.D, W.K-S., J.S-P.; resources, P.A and L.B.L.; data curation, P.A.; writing—original draft preparation, P.A.; writing—review and editing, L.B.L.; visualization, P.A.; supervision, P.A. and L.B.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experiments in this study comply with the current laws of Poland.
Consent for publication
The corresponding author declares, on behalf of all co-authors, that the work described is original, previously unpublished research, and not under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Izabela Pawłowicz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Figure S1.
UPGMA clustering of 60 Avena accessions based on the values of Dice similarity coefficient (PNG 550 kb)

Figure S2.
The composition of soluble carbohydrates in caryopses of studied Avena accessions (PNG 363 kb)
File S1.
Raw photographs of agarose gel electrophoresis for all studied Avena accesions and all applied PBS primers with description (RAR 17765 kb)
Table S1.
List of the Avena accessions used in the study. Species arranged alphabetically. (DOCX 18 kb)
Table S2.
Binary matrix of iPBS data for 60 Avena accessions. (XLSX 26 kb)
Table S3.
Dice similarity coefficient values between studied Avena accessions (XLSX 24 kb)
Table S4.
The content of soluble carbohydrates in caryopses of studied Avena accessions (XLSX 22 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Androsiuk, P., Milarska, S.E., Dulska, J. et al. The comparison of polymorphism among Avena species revealed by retrotransposon-based DNA markers and soluble carbohydrates in seeds. J Appl Genetics 64, 247–264 (2023). https://doi.org/10.1007/s13353-023-00748-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-023-00748-w