Abstract
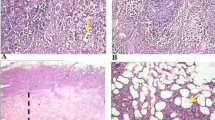
Salvia lachnostachys is an herbaceous plant with anti-inflammatory, analgesic and cytotoxic properties. This study investigated the antitumor effect of an ethanolic extract of Salvia lachnostachys leaves (EES) in a solid Ehrlich carcinoma model. Ehrlich cells were inoculated subcutaneously in the right pelvic member (2 × 106 cells) in female Swiss mice. The animals were treated with vehicle (10 mL kg−1, p.o.), EES (30 and 100 mg kg−1, p.o.), or methotrexate (2.5 mg kg−1, i.p.) for 21 days (early treatment) or 14 days (late treatment) after tumor inoculation, or 10 days before tumor inoculation and continued for 21 days after tumor inoculation (chemopreventive treatment). The acute toxicity test was performed according OECD guidelines Late treatment with EES had no antitumor effect. Early treatment with 100 mg kg−1 EES prevented tumor development, increased tumor necrosis factor-α (TNF-α) levels and decreased tumor superoxide dismutase (SOD) activity, interleukin-10 (IL-10) levels and Cyclin D1 expression, and tumor cell necrosis was observed. Chemopreventive treatment with EES for 10 and 31 days prevented tumor development in the same manner. EES treatment for 31 days decreased hepatic and tumor SOD activity, tumor IL-10 levels and Cyclin D1 expression, and increased tumor reduced glutathione, N-acetylglucosaminidase, reactive oxygen species, lipid peroxidation, TNF-α levels and Nrf2 expression. No toxicity was observed in the acute toxicity assay. In conclusion, EES had an antitumor effect by inhibiting Cyclin D1 expression and increasing inflammation with early and chemopreventive treatment. Modulation of the antioxidant system also contribute for the antitumor effects of EES.








Similar content being viewed by others
References
Black DJ, Livingston RB (1990) Antineoplastic drugs in 1990 a review (Part 1). Drugs 39:489–501. https://doi.org/10.2165/00003495-199039040-00002
Kinghorn AD, De Blanco EJC, Chai H, Orjala J, Farnsworth NR, Soejarto DD et al (2009) Discovery of anticancer agents of diverse natural origin. Pure Appl Chem 81:1051–1063. https://doi.org/10.1351/PAC-CON-08-10-16.Discovery
Erbano M, Ehrenfried CA, Stefanello MEA, Santos EP (2012) Morphoanatomical and phytochemical studies of Salvia lachnostachys (Lamiaceae). Microsc Res Tech 75:1737–1744. https://doi.org/10.1002/jemt.22125
Topçu G (2006) Bioactive triterpenoids from Salvia species. J Nat Prod. https://doi.org/10.1021/np0600402
Piccinelli AC, Aquino DFS, Morato PN, Kuraoka-Oliveira AM, Strapasson RLB, Santos EP et al (2014) Anti-inflammatory and antihyperalgesic activities of ethanolic extract and fruticulin A from Salvia lachnostachys leaves in mice. Evidence Based Complement Altern Med 2014:1–8. https://doi.org/10.1155/2014/835914
Oliveira CS, Salvador MJ, Carvalho JE, Santos EP, Barison A, Stefanello MEA (2016) Cytotoxic abietane-derivative diterpenoids of Salvia lachnostachys. Phytochem Lett 17:140–143. https://doi.org/10.1016/j.phytol.2016.07.005
Baillif RN (1954) The solid phase of the Ehrlich ascites tumor in mice. Cancer Res 14:554–558
Klein G (1950) Use of the Ehrlich ascites tumor of mice for quantitative studies on the growth and biochemistry of neoplastic cells. Cancer 3:1052–1061
Ozaslan M, Karagoz ID, Kilic IH, Guldur ME (2011) Ehrlich ascites carcinoma. Afr J Biotech 10:2375–2378
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200. https://doi.org/10.1038/1811199a0
Chen FA, Wu AB, Chen CY (2004) The influence of different treatments on the free radical scavenging activity of burdock and variations of its active components. Food Chem 86:479–484. https://doi.org/10.1016/j.foodchem.2003.09.020
OECD (2002) Test No. 423: acute oral toxicity—acute toxic class method. Oecd Guidel Test Chem. https://doi.org/10.1787/9789264071001-en
Malone MH (1977) Pharmacological approaches to natural product screening and evaluation. In: Wagner H, Wolff P (eds) New natural products and plant drugs with pharmacological, biological and therapeutics activity. Springer, Berlin, pp 23–53
Mishra S, Tamta AK, Sarikhani M, Desingu PA et al (2018) Subcutaneos Ehrlich ascites carcinoma mice model for studying cancer-induced cardiomyopathy. Sci Rep 8:5599. https://doi.org/10.1038/s41598-018-23669-9
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Jiang ZY, Woollard AC, Wolff SP (1991) Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 26:853–856. https://doi.org/10.1007/BF02536169
Gao R, Yuan Z, Zhao Z, Gao X (1998) Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Biochem Bioenerg 45:41–45. https://doi.org/10.1016/S0302-4598(98)00072-5
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Keston AS, Brandt R (1965) The fluorimetric analysis of ultramico quantities of hydrogen peroxide. Anal Biochem 11:1–5. https://doi.org/10.1016/0003-2697(65)90034-5
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138. https://doi.org/10.1016/0003-2697(82)90118-X
Bailey PJ (1988) Sponge implants as models. Methods Enzym 162:327–334. https://doi.org/10.1016/0076-6879(88)62087-8
Machado CL, Pasini FS, Mangone FRR, Katayama MLH, Roela RA, Maxxotii TKF, Chammas R (2015) Protocolos teóricos práticos em Biologia Molecular aplicada a câncer. Biologia molecular: da teoria à prática—Determinando expressão gênica e variações na sequência de DNA. In: Saito RF, Lana MVG, Medrano RFV, Chammas R (eds) Fundamentos da oncologia molecular. São Paulo, Atheneu, pp 447–453
Bisio A, Romussi G, Russo E, Cafaggi S, Schito AM, Repetto B et al (2008) Antimicrobial activity of the ornamental species Salvia corrugata, a potential new crop for extractive purposes. J Agric Food Chem 56:10468–10472. https://doi.org/10.1021/jf802200x
Wang M, Li J, Rangarajan M, Shao Y, La Vole EJ, Huang T et al (1988) Antioxidative phenolic compounds from sage (Salvia officinalis). J Agric Food Chem. https://doi.org/10.1021/jf980614b
Akaberi M, Mehri S, Iranshahi M (2015) Multiple pro-apoptotic targets of abietane diterpenoids from Salvia species. Fitoterapia 100:118–132. https://doi.org/10.1016/j.fitote.2014.11.008
Gonzalez MA (2015) Aromatic abietane diterpenoids: their biological activity and synthesis. Nat Prod Rep 32:684–704. https://doi.org/10.1039/c4np00110a
Di Domenico F, Foppoli C, Coccia R, Perluigi M (2012) Antioxidants in cervical cancer: chemopreventive and chemotherapeutic effects of polyphenols. Biochim Biophys Acta 1822:737–747. https://doi.org/10.1016/j.bbadis.2011.10.005
Miranda-Vilela AL, Portilho FA, Araujo FGB, Estevanato LLC, Mezzomo BP, Fátima M (2011) The protective effects of nutritional antioxidant therapy on Ehrlich solid tumor- bearing mice depend on the type of antioxidant therapy chosen: histology, genotoxicity and hematology evaluations. J Nutr Biochem 22:1091–1098. https://doi.org/10.1016/j.jnutbio.2010.09.009
Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J et al (2015) Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol 35:1–30. https://doi.org/10.1016/j.semcancer.2015.02.006
Goetz M, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J et al (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S et al (2016) Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med 375:1738–1748. https://doi.org/10.1056/NEJMoa1609709
Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A et al (2017) NeoPalAna: neoadjuvant pabociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor positive breast cancer. Clin Cancer Res 23:4055–4065. https://doi.org/10.1158/1078-0432.CCR-16-3206.NeoPalAna
Zhong H, Yin H (2015) Redox biology role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol 4:193–199. https://doi.org/10.1016/j.redox.2014.12.011
Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL (2009) Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390:191–214. https://doi.org/10.1515/BC.2009.033.Glutathione
Corso CR, Acco A (2018) Glutathione system in animal model of solid tumors: from regulation to therapeutic targets. Crit Rev Oncol Hematol 128:43–57. https://doi.org/10.1016/j.critrevonc.2018.05.014
Hayes JD, McMahon M (2006) The double-edged sword of Nrf2: subversion of redox homeostasis during the evolution of cancer. Mol Cell 21:732–734. https://doi.org/10.1016/j.molcel.2006.03.004
Milkovic L, Zarkovic N, Saso L (2017) Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox Biol 12:727–732. https://doi.org/10.1016/j.redox.2017.04.013
Roberts NJ, Zhou S, Diaz LAJ, Holdhoff M (2011) Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget 2:739–751. https://doi.org/10.18632/oncotarget.344
Brennan FM, Green P, Amjadi P, Robertshaw HJ, Takata M (2008) Interleukin-10 regulates TNF-alpha– converting enzyme (TACE/ADAM-17) involving a TIMP-3 dependent and independent mechanism. Eur J Immunol 38:1106–1117. https://doi.org/10.1002/eji.200737821
Ofengeim D, Yuan J (2013) Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol 14:727–736. https://doi.org/10.1038/nrm3683
Abdel-daim MM, Khalifa HA, Abushouk AI, Dkhil MA, Al-quraishy SA (2017) Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: a biochemical and histopathological study in mice. Oxid Med Cell Longev 2017:1–10. https://doi.org/10.1155/2017/3281670
Patel NN, Ghodasara DJ, Pandey S, Ghodasara PD, Khorajiya JH, Joshi BP et al (2015) Subacute toxicopathological studies of methotrexate in Wistar rats. Vet World 7:489–495. https://doi.org/10.14202/vetworld.2014
Spinella R, Sawhney R, Jalan R (2016) Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int 10:124–132. https://doi.org/10.1007/s12072-015-9665-6
Acknowledgements
We thank LCM, TBS, JPA, and RSC for their help with the experiments, ÉPS for plant collection, CRSS from the Brasilia University (UnB) for providing the Ehrlich cells, Prof. Dr. Rosangela L. Dittrich of VH/UFPR for helping with the hematological analysis, and CTAF-UFPR.
Funding
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES—Finance Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq-No. 307977/2015-3).
Author information
Authors and Affiliations
Contributions
CRC was responsible for experimental design, experiments execution, statistical analysis and writing; MCS and ERA helped in experiments with mice; LMS, MM and SFA conducted cytokine measurements; EASR and GK conducted qPCR experiment; OCB conduct hematological analysis; JEQT helped in histological analysis; CSO and MEAS provided plant extract; and AA was responsible for data analysis and critically revised the article. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants. The experiments performed with mice followed international rules for laboratory animal use and were approved by the Ethics Committee of Animal Experimentation (CEUA/BIO, UFPR; no. 879). Environmental enrichment was used in all cages along experiments to improve the mice welfare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Corso, C.R., Stipp, M.C., Adami, E.R. et al. Salvia lachnostachys Benth has antitumor and chemopreventive effects against solid Ehrlich carcinoma. Mol Biol Rep 46, 4827–4841 (2019). https://doi.org/10.1007/s11033-019-04931-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04931-3