Abstract
Astraea is a genus with 13 species, widely distributed throughout Neotropics and especially diverse in Brazil. The genus is currently placed in Crotoneae as sister to Acidocroton. This work aimed to characterize the pollen of Astraea as a contribution to the taxonomy and to understand the evolution of pollen traits between Astraea and Acidocroton. Pollen grains of 12 species of Astraea and three of Acidocroton were gathered from herbarium specimens, acetolyzed, measured, photographed and described under light microscope and scanning electron microscopy. Our results demonstrate that Astraea is a stenopolinic (stenopalynous) genus with pollen grains that are apolar, spherical, medium to large, inaperturate, showing a Croton pattern exine with sexine thicker than nexine, and with rosettes having clavae or pila in their lumen. The rosettes consist of 5-7(-8) pila that can be rounded to triangular with psilate to plicate surface. Pollen features did not reveal synapomorphies for Astraea, Acidocroton or even for the Astraea + Acidocroton clade. Several pollen features evolved independently among recent lineages of Astraea, and we interpreted these evolutionary shifts as adaptations to new habitats. This work consolidates the palynological knowledge of Astraea and Acidocroton and will contribute to future palynological and systematic studies in Euphorbiaceae.
Similar content being viewed by others
Introduction
Astraea Klotzsch is a genus in Euphorbiaceae represented by 13 species that are found in the Neotropics and Tropical West Africa, with its greatest diversity in eastern Brazil (Silva et al. 2020). Weedy species, such as A. lobata (L.) Klotzsch and A. trilobata (Forssk.) O.L.M.Silva & Cordeiro, are found in the Old World (Silva et al. 2019). Species of Astraea may be recognized by their mostly deeply lobed or partite leaves, spiciform thyrses, staminate flowers with petals bearing moniliform trichomes at the base and stamens incurved in bud, pistillate flowers with slender, cylindrical multifid styles and mostly tetrangular seeds in cross section (Silva et al. 2020).
The genus Astraea is currently placed in tribe Crotoneae, subfamily Crotonoideae (Wurdack et al. 2005; Berry et al. 2005; Webster 2014), the most species rich lineage of Euphorbiaceae in the Neotropics (Ulloa-Ulloa et al. 2017). In spite of being traditionally included as a section of the giant Croton L. (Baillon 1858; Webster 1993), molecular data place Astraea as sister to Acidocroton Griseb. (Berry et al. 2005; Silva et al. 2020), found in Antilles, Central America, and northern South America. Berry et al. (2005) showed that the Astraea-Acidocroton clade emerged sister to the hyperdiverse Brasiliocroton-Croton clade, but a recent phylogeny recovered Croton sister to the Astraea-Acidocroton clade (Silva et al. 2020). The close relationship between Astraea and Acidocroton is surprising since the latter has distinctive xerophytic features in Crotoneae, such as stipules transformed in spines, leaves mostly arranged in short shoots (brachiblasts) and glomeruliform inflorescences (Webster 2014).
Pollen data have long been used in the classification of Euphorbiaceae (Punt 1962; Webster 1975, 1994, 2014). Crotoneae pollen has been studied extensively, but most works focused only on Croton (Erdtman 1952; Punt 1962; Nowicke 1994; Lobreau-Callen and Cervera 1997; Souza et al. 2016, 2019). For Astraea, only two works include descriptions of pollen grains (Oliveira and Santos 2000, Carreira and Barth 2003), whereas for Acidocroton no pollen data exist.
Taxonomic changes were proposed recently for Astraea including the re-delimitation of A. lobata (Silva et al. 2019), recognition of distinct species based on former varieties of Croton lobatus (Silva and Cordeiro 2017, Silva et al. 2019) and revision of the delimitation of some species, such as A. paulina and A. cincta (Silva et al. 2017, 2019). The genus is monophyletic and consists of three main clades (Silva et al. 2020). As palynological data are scarce for the genus, we aimed to characterize the pollen grains of Astraea, including a few representatives of Acidocroton, as a contribution to their taxonomy, to identify synapomorphies for both clades, and finally to infer the evolution of pollen traits within Astraea using the most recent phylogeny.
Materials and methods
Taxon sampling
Samples of 40 specimens from 12 of the 13 species of Astraea, plus five specimens from three of the 12 species of Acidocroton (Table 1) were gathered from collections deposited in ALCB, CEN, COL, G, HUEFS, RB, SP, SPF, MBM, MO, UB, US and VIC.
Light (LM) and scanning electronic microscopy (SEM)
For LM, pollen grains were submitted to acetolysis following Erdtman (1960). From five of the mounted slides, one was stained with safranin. Slides were identified and deposited in the pollen library at Plant Micromorphology Laboratory of the Universidade Federal de Feira de Santana, Bahia, Brazil.
For SEM, acetolyzed pollen grains were washed and dehydrated in ascending hydroethanolic series (50, 70, 90 and 100%), for 10 min at each step. A drop of absolute ethanol containing pollen grains was directly applied to a stub and, after complete drying, the sample was metalized with high-vacuum gold evaporation. Samples were then qualitatively analyzed, and images were obtained using a Quanta 250 microscope (FEI Company) at the Electronic Microscopy Center in the Universidade Estadual de Santa Cruz (UESC) and a JEOL 6390LV microscope at the Plataforma de Microscopia Eletrônica in Centro de Pesquisas Gonçalo Moniz (FIOCRUZ).
Pollen grains characterization
Pollen grains were described regarding size, shape, polarity, apertures, ornamentations and exine sculpture, adopting the nomenclature of Punt et al. (2007) and Hesse et al. (2009). The size classes follow Erdtman (1952). The measures were taken randomly in 25 pollen grains for each specimen, distributed in, at least, five slides. Exine thickness and diameter of pila, rosettes and the central space within rosettes were measured in 10 pollen grains from each specimen as illustrated by Souza et al. (2016).
Quantitative data were treated statistically, with arithmetic mean, sample and mean standard deviation, coefficient of variability, 95% confidence interval and variation range for measures taken from 25 pollen grains for each specimen. For exine thickness and diameter of pila, rosettes and the central space within rosettes we only calculated the arithmetic mean.
Ancestral states reconstruction
We employed the phylogeny of Astraea resulted from Bayesian inference based on ITS sequences from all Crotoneae genera from Silva et al. (2020), deposited in TreeBase (study ID 25606). As our study focuses on Astraea, we used Acidocroton as outgroup, and the remaining genera of Crotoneae were pruned from the tree using the drop.tip function from the ape package (Paradis and Schliep 2019) in R (R Core Team 2020). Six palynological characters (Table 2) were selected and their states coded and treated as unordered. Reconstructions of ancestral states were then performed using maximum likelihood, with one-parameter Markov k-state (Mk1) model, as implemented in Mesquite v. 3.2 (Maddison and Maddison 2009).
Results
Pollen grains characterization
Astraea
Pollen grains of Astraea are monads, apolar, spherical, inaperturate, with Croton pattern exine, and sexine thicker than the nexine (Figs. 1, 2, 3, 4). The predominant size is medium (Tables 3 and 4, Figs. 5 and 6), but large pollen grains are also observed exclusively in A. cincta (Müll.Arg.) Caruzo and Cordeiro. In Astraea, the rosette has 5-7(-8) pila with subcircular to subtriangular heads, which lumen has sexine elements. In A. cincta (Fig. 1c) and A. surinamensis (Miq.) O.L.M.Silva & Cordeiro (Fig. 4l), the lumen of the rosettes is reduced.
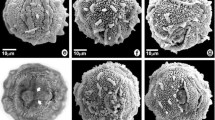
Pollen grains of species of Astraea (Euphorbiaceae). Astraea cincta: a optical section; b detail of the surface; c surface (SEM); A. lobata: d optical section; e detail of the surface; f surface (SEM); A. macroura: g optical section; h detail of the surface; i surface (SEM); A. surinamensis: j optical section; k detail of the surface; l surface (SEM). Scale bars 5 μm (b, e, h, k), 10 μm (a, c–d, f–g, i–j, l)
Pollen grains of species of Astraea (Euphorbiaceae). Astraea comosa: a optical section; b detail of the surface; c surface (SEM); A. digitata: d optical section; e detail of the surface; f surface (SEM); A. jatropha: g optical section; h detail of the surface; i surface (SEM); A. paulina: j optical section; k detail of the surface; l surface (SEM). Scale bars 5 μm (b, e, h, k) 10 μm (a, c–d, f–g, i–j, l)
Pollen grains of species of Acidocroton and Astraea (Euphorbiaceae). Acidocroton gentryi: a optical section; b detail of the surface; c surface (SEM); Astraea cincta: d detail of exine structure (SEM), arrow showing clavae; Astraea comosa: e detail of the surface (SEM); Astraea gracilis: f detail of exine structure (SEM); Astraea lobata: g detail of the surface (SEM); Astraea praetervisa: h detail of the surface (SEM); Astraea surinamensis: i detail of the surface (SEM). c, clava; p, pilum; arrows indicate sexine elements in the lumen of the rosettes. Scale bars 5 μm (b–i), 10 μm (a)
SEM details of the surface of pollen grains of species of Acidocroton and Astraea (Euphorbiaceae). aAcidocroton gentryi; bAstraea cincta; cAstraea comosa; dAstraea digitata; eAstraea klotzschii; fAstraea lobata; gAstraea macroura; hAstraea jatropha; iAstraea paulina; jAstraea praetervisa; kAstraea subcomosa; lAstraea surinamensis. Scale bars 2 μm (c, e–f, h–l), 5 μm (a, b, d, g)
Boxplot graph of the distribution of the variable diameter of pollen grains of Acidocroton and Astraea (Euphorbiaceae). The horizontal bar inside the rectangle is the median, the rectangle shows 50% of interquartile, the ends show the amplitude variation, and the black circles correspond to the outlier
Pilum surface varies from psilate in Astraea comosa (Müll.Arg.) B.W. van Ee (Fig. 4c), A. klotzschii Didr. (Figure 4e), A. paulina Didr. (Fig. 4i) and A. subcomosa (Müll.Arg.) Caruzo (Fig. 4k), to plicate in the remaining species of Astraea (Fig. 4a–b, d, f–h, j, l). In the later, plicae show different levels of folding density (number) and deepness of the pilum grooves (Fig. 4). Only in A. cincta and A. digitata (Müll.Arg.) O.L.M.Silva & Cordeiro, we observed more than 10 plicae per pilum. As for deepness, only in A. cincta and A. praetervisa (Müll.Arg.) P.E.Berry, shallow folding is observed. Overall, pila with shallow plicae show more foldings than those with deep plicae: a minimum of eight foldings in deep plicae and a maximum of 13 in shallow plicae.
The sexine elements of the rosette lumen are clavae or pila (Figs. 1, 2, 3, 4). Almost all species have only one of them (Fig. 3d; Table 3), while in A. lobata and A. comosa, have both (Fig. 3g). The distribution of clavae and pila vary from dense in A. gracilis (Müll.Arg.) O.L.M.Silva & Cordeiro, A. macroura (Mart. ex Colla) P.L.R.Moraes, De Smedt & Guglielmone (Fig. 4g), A. jatropha (Müll.Arg.) B.W.van Ee (Fig. 4h), A. praetervisa (Fig. 4j) and A. subcomosa (Fig. 4k) to slightly sparse in the remaining species.
In the lumen of rosettes, clavae and pila are sparse surrounding the base of the pilum in most species (Fig. 4c, g, k) or aggregated in the central region of the lumen in A. gracilis, A. jatropha and A. praetervisa (Fig. 4h, j). Finally, the sexine is thicker than the nexine in all species we analyzed (Table 3), with the thinnest in A. digitata (2.7 μm) and the thickest in A. cincta (4.0 μm).
Acidocroton
Pollen grains of Acidocroton are monads, apolar, spherical, inaperturate, with Croton pattern exine and sexine thicker than the nexine (Figs. 3a–c, 4a). Species of Acidocroton have medium pollen grains with diameter varying from 33.8 μm in A. gentryi Fern.Alonso & R.Jaram to 44.1 μm in A. oligostemon Urb. (Table 4, Fig. 5). The rosette is composed by 5-7 triangular pila, or subtriangular in A. gentryi and A. oligostemon. In A. gentryi, pilum surface is plicate, pila with deep plicae. The rosettes have a well delimited lumen with clavate sparsely distributed surrounding the base of the pilum in A. gentry to aggregate in the central region of the lumen in the other species.
Ancestral states reconstruction
Our ancestral state reconstruction (Fig. 7) indicates that the putative ancestor of Astraea most likely had medium pollen grains bearing subcircular pila and spaced sexine elements in the lumen of the rosettes surrounding the base of the pilum and with sexine of 3–4 μm. For the pilum surface, our reconstruction could not recover a most likely state between plicate and psilate.
Inferred ancestral state reconstruction of Acidocroton and Astraea (Euphorbiaceae) for pollen size, pilum head shape, pilum surface, distribution and disposition of sexine elements in the lumen of the rosettes and sexine thickness using maximum likelihood over the ITS tree from Silva et al. (2020). Color codes for character states are shown on the upper left; gray represents unknown or inconclusive state reconstruction. Character state shifts in putative ancestors are indicated with a red arrow
Regarding pollen grains size, we recovered transitions to slightly larger pollen grains (from less than 40 μm to up to 46 μm) only in species of clade C (A. comosa, A. jatropha, A. klotzschii, A. paulina and A. subcomosa) and to more than 50 μm in A. cincta. Most putative ancestors in Astraea were inferred as having subcircular pilum head, with a shift to subtriangular ones in the putative ancestor of clade C2, which could represent a synapomorphy for this group, although A. digitata has also subcircular pilum head. In this scenario, the subtriangular pilum head, observed also in A. macroura, has an independent origin of this feature in C1, and circular pilum head, observed only in A. comosa and A. subcomosa, is restricted to C1. Nevertheless, the psilate pilum surface evolved separately in C1 (A. subcomosa and A. comosa) and C2 (A. paulina and A. klotzschii).
While most putative ancestors in Astraea were inferred as having sparse sexine elements in the lumen of the rosette, for clade C1 we inferred a putative ancestor with dense sexine elements in the lumen of the rosette. This shift could be interpreted as a synapomorphy for C1, with a reversion to sparse sexine elements in A. comosa. Regarding the disposition of such sexine elements, independent shifts from spaced, surrounding the base of the pilum, to aggregate in the central region of the rosette, were inferred in both C1 (A. jatropha and A. praetervisa) and C2 (A. gracilis). Finally, for sexine thickness, we inferred independent reductions (to up to 2.9 μm) in A. lobata and both C1 (A. praetervisa) and C2 (A. digitata and A. gracilis), with the sexine with more than 4.0 μm, as well as the pollen larger than 50 μm, representing autapomorphies of A. cincta.
Discussion
The work of Carreira and Barth (2003) described the pollen grains of Astraea paulina (as Croton lobatus in their work) as slightly large (48–53 μm), while Oliveira and Santos (2000) described the pollen of A. surinamensis (as C. lobatus in their work) similar to what we observed. Our analyzes showed that the majority of Astraea pollen grains are in the 40–50 μm range. The smallest diameter was observed in Acidocroton gentryi (33.8 μm), and Astraea cincta has the largest pollen grains (51.9 μm). Although we could not identify any synapomorphy for the clade Astraea + Acidocroton, our work confirmed that spheroidal and inaperturate pollen grains with Croton pattern ornamentation are shared among all representatives of Crotoneae following the circumscription of Wurdack et al. (2005). The presence of sexine elements in the lumen of the rosettes, as well as having the sexine thicker than the nexine, are features widely present in Croton, Brasiliocroton and Sagotia (Erdtman 1952; Thanikaimoni et al. 1984; Nowicke 1994; Lima et al. 2007; Souza et al. 2016, 2019) and now extended to Acidocroton and Astraea because of our study.
The morphology of the pilum surface of the rosette is a character of taxonomic value as demonstrated by Thanikaimoni et al. (1984), Nowicke (1994), Carreira et al. (1996), Lobreau-Callen and Cervera (1997), Souza et al. (2016), Ren-Yong et al. (2018) and Souza et al. (2019). Carreira et al. (1996), who studied the pollen morphology of several lianescent species of Croton, verified the presence of pilum with a striate and psilate surface. Ren-Yong et al. (2018) showed that the Trigonostemon (Euphorbiaceae) species differ from other genera (Dimorphocalyx, Ostodes and Tritaxis) by the absence of striate ornamentation on the subunits (pila). Souza et al. (2019) showed that the species of the genus Brasiliocroton have pila with variation in the surface of ornamentation, Brasiliocroton mamoninha P.E.Berry & Cordeiro has pila > 5-plicate and pointed apex, whereas B. muricatus Riina & Cordeiro had pila < 5-Plicate and rounded apex, thus contributing to the taxonomic circumscription of these taxa. For Thanikaimoni et al. (1984), the ornamentation of sculptural elements (pilum) is a diversification of the Croton pattern and may also be associated with pollination. In Astraea, however, we observed only psilate and plicate pila.
Most pollen characters analyzed are conserved in Astraea, with shifts concentrated within clades and evolving independently, a pattern also documented for monocots and basal angiosperms (Lu et al. 2015, Zhang et al. 2017). Silva et al. (2020) inferred recent divergences (from 10 Ma onward) within clades A, B, C1 and C2 of Astraea, most likely influenced by the colonization of humid areas, as, for example, in clade C1 we find species restricted to dry (A. comosa and A. subcomosa in campo rupestres) and humid areas (A. macroura and A. praetervisa in seashore vegetation). Therefore, shifts in pollen features may also be linked to the colonization of new habitats along the evolutionary history of Astraea.
Psilate pila are inferred as derived states (Fig. 7) in two distinct lineages: Astraea comosa and A. subcomosa in C1 and A. klotzschii and A. paulina in C2. These species with psilate pila are the only ones found in campos rupestres (Silva et al. 2020): A. comosa is restricted to the Meridional Espinhaço Range, while A. subcomosa is restricted to the Septentrional Espinhaço Range and Chapada Diamantina, A. paulina is found along the Espinhaço Range, but also in gallery forests in the Cerrado domain, and A. klotzschii is mainly found in seashore vegetation in eastern Brazil, but with a few collections from Chapada Diamantina.
Astraea cincta is unique in the genus due to its xylopodiferous underground system, cartilaginous leaf margin and rounded seeds (Silva et al. 2019). Pollen data provided additional unique features for this species: the largest pollen grains and the thickest exine (Fig. 7; Table 3). Such features seem to be related to preventing water loss during pollen dispersal as Nepi et al. (2001) have suggested for many groups of angiosperms. In fact, A. cincta is found in seasonally dry open vegetation in central Brazil and eastern Bolivia (Silva et al. 2019). Other factors such as transport conditions and level of polyploidy may reflect directly in pollen size (Muller 1979). However, there are no data available in the literature for cytotaxonomy and pollination system involving Astraea species, except the chromosome number of A. lobata (Miller and Webster 1966).
Medium pollen grains are observed in the remaining species of Astraea. However, most species restricted to the Atlantic rain Forest domain (namely, A. gracilis, A. digitata, A. macroura and A. praetervisa) have slightly smaller pollen grains (35.8–40.6 μm vs 43–48 μm in the remaining species with medium pollen grains), indicating that smaller pollen grains in Astraea may be associated with humid areas in both C1 and C2. In C2, A. gracilis and A. digitata have congruent patterns also in pilum surface and exine thickness, when compared to their sister species (A. paulina and A. klotzschii). The occurrence of Astraea paulina in the Cerrado domain suggests an association of plicate pilum surface and thinner exine with more humid habitats. Although A. klotzschii is also in the Atlantic Forest, it occurs in a different habitat (mostly restricted to seashore vegetation) from A. gracilis and A. digitata (borders of humid forests).
Finally, in support of the segregation of Astraea digitata, A. gracilis, A. jatropha and A. surinamensis from A. lobata, our data show that only A. lobata has both pilum and clava as sexine elements inside the lumen of its rosettes, while the remaining species have strictly clavate sexine elements. Also, A. jatropha and A. digitata have a high number of pilum head folding (10 and 13, respectively, vs. eight in A. lobata), while A. digitata and A. gracilis have smaller pollen grains (38.2 and 40.6 μm, respectively, vs. 45.4 μm in A. lobata) with slightly thinner sexine (2.7 and 2.8 μm, respectively, vs. 3.2 μm in A. lobata). Pollen grains of A. surinamensis, however, are very similar to those of A. lobata.
Conclusions
With an almost complete sampling of Astraea, we found little variation in pollen morphology, confirming its stenopalynous nature. Our pollen data support the placement of Astraea in Crotoneae based on shared features such as apolar, spheroidal, inaperturate pollen grains with Croton pattern exine. Shifts to less conserved pollen features in the most recent diverging clade of Astraea may be associated with the colonization of new habitats. Distinct pollen features among species within that clade also suggest this pattern. Although we did not identify any synapomorphy, neither for Astraea nor for the Astraea + Acidocroton clade, our results expand the palynological data for Crotoneae and will contribute to future taxonomic and phylogenetic studies in this group.
References
Baillon HE (1858) Étude générales du groupe des Euphorbiacées. Librairie de Victor Masson, Paris
Berry PE, Hipp AL, Wurdack KJ, Ee BV, Riina R (2005) Molecular phylogenetics of the giant genus Croton and tribe Crotoneae (Euphorbiaceae sensu stricto) using its and trnl-trnf DNA sequence data. Amer J Bot 92:1520–1534. https://doi.org/10.3732/ajb.92.9.1520
Carreira LMM, Barth OM (2003) Atlas de Pólen da vegetação de Canga da Serra de Carajás. Museu Paraense Emílio Goeldi. Pará, Brasil, pp 37–43
Carreira LMM, Secco RS, Barth OM (1996) Pollen morphology of the lianescent species of the genus Croton (Euphorbiaceae). Grana 35:74–78. https://doi.org/10.1080/00173139609429476
Erdtman G (1952) Pollen morphology and plant taxonomy: angiosperms. Almqvist and Wiksell, Stockholm
Erdtman G (1960) The acetolysis method. A revised description. Svensk Bot Tidskr 54:561–564
Hesse M, Halbritte H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S (2009) Pollen terminology: an illustrated handbook. Springer, New York
Lima LR, Cruz-Barros MAV, Pirani JR, Corrêa AMS (2007) Pollen morphology of Croton sect. Lamprocroton (Mull.Arg.) Pax (Euphorbiaceae) and its taxonomic implications. Nordic J Bot 25:206–216. https://doi.org/10.1111/j.2007.0107-055X.00076.x
Lobreau-Callen D, Cervera MS (1997) Le pollen des Crotonoideae Apétales (Euphorbiaceae): Ultrastructure de l’exine. Pollen exine ultrastructure of the apetalous Crotonoideae. Rev Palaeobot Palynol 98:257–291. https://doi.org/10.1016/S0034-6667(97)00012-2
Lu Lu AHW, De-zhu Li HW, Blackmore S (2015) Evolution of angiosperm pollen. 2. The basal angiosperms. Ann Missouri Bot Gard 100:177–226. https://doi.org/10.3417/2012047
Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis. Version 2.72. Available at: http://mesquiteproject.org. Accessed 10 June 2019
Miller KI, Webster GL (1966) Chromosome numbers in the Euphorbiaceae. Brittonia 18:372–379. https://doi.org/10.2307/2805153
Muller J (1979) Form and function in angiosperm pollen. Ann Missouri Bot Gard 66:593–632. https://doi.org/10.2307/2398913
Nepi M, Franchi GG, Pacini E (2001) Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma 216:171–180. https://doi.org/10.1007/BF02673869
Nowicke JW (1994) A palynological study of Crotonoideae (Euphorbiaceae). Ann Missouri Bot Gard 81:245–269. https://doi.org/10.2307/2992096
Oliveira PP, Santos FAR (2000) Morfologia polínica do gênero Croton L. (Euphorbiaceae) dos inselbergs da região de Milagres (Bahia-Brasil). Revista Univ Guarulhos Geoci 5:212–215
Paradis E, Schliep K (2019) Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. https://doi.org/10.1093/bioinformatics/bty633
Punt W (1962) Pollen morphology of the Euphorbiaceae with special reference to taxonomy. Wentia 7:1–116. https://doi.org/10.1111/j.1438-8677.1962.tb00010.x
Punt W, Hoen PP, Blackmore S, Nilson S, Le Thomas A (2007) Glossary of pollen and spore terminology. Rev Palaeobot Palynol 143:1–81. https://doi.org/10.1016/j.revpalbo.2006.06.008
R Core Team (2020) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Avaliable at: http://www.r-project.org/index.html. Accessed 10 june 2019
Ren-Yong Y, Van Der Ham R, Welzen PV (2018) Pollen morphology of Trigonostemon and its relatives (Euphorbiaceae). Grana 58:114–128. https://doi.org/10.1080/00173134.2018.1536763
Silva OLM, Cordeiro I (2017) Disentangling Astraea lobata: three new taxa in Astraea based on previous varieties of Croton lobatus (Euphorbiaceae). Phytotaxa 317:297–300. https://doi.org/10.11646/phytotaxa.317.4.5
Silva OLM, Banzato TC, Bedendo IP, Cordeiro I (2017) A report of infestation by phytoplasmas in Astraea (Euphorbiaceae) and its taxonomic implications in Astraea douradensis. Phytotaxa 332:195–198. https://doi.org/10.11646/phytotaxa.332.2.7
Silva OLM, Dias P, Riina R, Cordeiro I (2019) Redelimitation of Astraea lobata (Euphorbiaceae) and other taxonomic rearrangements in Astraea. Phytotaxa 404:127–136. https://doi.org/10.11646/phytotaxa.404.4.1
Silva OLM, Riina R, Cordeiro I (2020) Phylogeny and biogeography of Astraea with new insights into the evolutionary history of Crotoneae (Euphorbiaceae). Molec Phylogen Evol 145:106738. https://doi.org/10.1016/j.ympev.2020.106738
Souza LR, Carneiro-Torres DS, Saba MD, Santos FAR (2016) Pollen morphology of Crotonoideae (Euphorbiaceae) from seasonally dry tropical forests, Northeastern Brazil. Pl Syst Evol 302:795–817. https://doi.org/10.1007/s00606-016-1300-z
Souza LR, Santos FAR, Carneiro-Torres DS (2019) Pollen morphology and exine ultrastructure of Brasiliocroton P. E. Berry and Cordeiro (Euphorbiaceae). Acta Bot Brasil 33:584–591. https://doi.org/10.1590/0102-33062019abb0183
Thanikaimoni G, Caratini C, Nilsson S, Grafström E (1984) Omniaperturate Euphorbiaceae pollen with striate spines. Bull Jard Bot Natl Belg 54:105–125. https://doi.org/10.2307/3667867
Ulloa-Ulloa C, Acevedo-Rodriguez P, Beck S, Belgrano MJ, Bernal R, Berry PE, Brako L, Celis M, Davidse G, Forzza RC, Gradstein SR, Hokche O, León B, León-Yánez S, Magill RE, Neill DA, Nee M, Raven PH, Stimmel H, Strong MT, Willaseñor JL, Zarucchi JL, Zuloaga FO, Jorgensen PM (2017) An integrated assessment of the vascular plant species of the Americas. Science 358:1614–1617. https://doi.org/10.1126/science.aao0398
Webster GL (1975) Conspectus of a new classification of the Euphorbiaceae. Taxon 24:593–601. https://doi.org/10.2307/1220725
Webster GL (1993) A provisional synopsis of the sections of the Genus Croton (Euphorbiaceae). Taxon 42:793–823. https://doi.org/10.2307/1223265
Webster GL (1994) Classification of the Euphorbiaceae. Ann Missouri Bot Gard 81:3–32. https://doi.org/10.2307/2399908
Webster GL (2014) Euphorbiaceae. In: Kubitzki K (ed) The families and genera of vascular plants, 11th edn. Springer, Berlin, pp 51–216
Wurdack KJ, Hoffmann P, Chase MW (2005) Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using plastid rbcl and trnl-f DNA sequences. Amer J Bot 92:1397–1420. https://doi.org/10.3732/ajb.92.8.139714
Zhang MY, Lu Lu AHW, De-Zhu Li HW, Blackmore S (2017) Evolution of angiosperm pollen: 4. Basal eudicots. Ann Missouri Bot Gard 102:141–182. https://doi.org/10.3417/2015035
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa dos Estados da Bahia (FAPESB) and São Paulo (FAPESP) for the scholarship awarded to LRS (#2018/3046) and OLMS (#2013/26501-6 and #2017/06171-2), respectively; to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support to FARS (#302594/2016-7) and IC (#309917/2015-8); to the curator of the visited herbaria for providing access to their collections; and to the Laboratório de Palinologia from Universidade do Estado da Bahia—Campus VII.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Handling Editor: Ricarda Riina.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, L.R., Carneiro-Torres, D.S., da Silva, O.L.M. et al. Pollen morphology and evolution in Astraea (Euphorbiaceae: Crotoneae). Plant Syst Evol 306, 55 (2020). https://doi.org/10.1007/s00606-020-01683-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-020-01683-7