Abstract
Organic N (oN, e.g., amino acids) is an important N-resource for plants in soils replete with oN but not inorganic N (iN; i.e., NH4+ and NO3−), such as cold ecosystems with temperature-limited soil decomposition rates. However, sub-Antarctic literature assumes that plants only acquire iN, potentially underestimating plant-available N. We hypothesised that Marion Island (− 46.90°, 37.75°) grasses (Polypogon magellanicus, Poa cookii, Agrostis stolonifera and Poa annua) acquire oN and that oN relative to iN provision affects plant growth. We investigated oN and iN uptake and growth responses in two hydroponics experiments. In situ N (15N-glycine, 15NO3− and 15NH4+) acquisition was investigated at three field sites with decreasing faunal influence, thus iN input and microbial activity. When plants grown in mire water were supplied with 15N-glycine or 15NO3−, root δ15N enrichment was highest for glycine-supplied plants. In the second hydroponics experiment, plant N-uptake rates (nmol g biomass−1 s−1) were significantly higher for glycine than NO3−, but relative growth rates (g g−1 d−1) lower on glycine. There were species-specific biomass allocation responses to N concentration (4 mM and 0.4 mM) and N-form (glycine and NO3−). Glycine-supplied grasses at the low iN concentration field sites had significantly higher δ15N enrichment relative to those at sites with high iN, suggesting higher oN uptake when iN is limiting. We demonstrate the importance of accounting for oN acquisition in the sub-Antarctic. As a system with high soil oN relative to iN, plants may predominantly meet N-demands through oN rather than iN acquisition.
Similar content being viewed by others
Introduction
Plant productivity is a key component of the terrestrial N-cycle, and thus identifying plant-available N sources substantially influences our understanding of it. Until the late twentieth century, the soil N-cycle was understood to be regulated by microbial mineralisation of soil organic matter (SOM) (Schimel and Bennett 2004). Most literature assumed inorganic N (iN; i.e., NH4+ and NO3−) was the only N source ecologically relevant for plant acquisition (Smith and Steenkamp 1992a), despite the capacity for direct organic N (oN, e.g., free amino acids) acquisition being long established (e.g., Hutchinson & Miller 1912; Virtanen & Linkola 1946; Wright 1962). However, instead of microbial mineralisation, the soil N-cycle is largely driven by the depolymerisation of SOM by extracellular enzymes, which release oN monomers and polymers for acquisition by both plants and microbes (Schimel and Bennett 2004). This challenges two important assumptions in N-cycling literature. First, that microbes are always superior competitors for oN compared to plants, with evidence for successful in situ plant competition for oN (Lipson and Monson 1998; Hodge et al. 2000; Schmidt et al. 2002). Secondly, and the focus of this paper, that plants rely only on iN pools to meet their N-requirements.
Plants can acquire oN molecules both directly, mediated by root transporters, or through mycorrhizal symbionts (Miller and Cramer 2004; Näsholm et al. 2009; Acuña-Rodríguez et al. 2020). There is widespread evidence for plant oN acquisition, spanning a range of ecosystems. For example, oN acquisition has been documented in Arctic tundra (Kielland 1994, 2001; Nordin et al. 2004), the Antarctic (Hill et al. 2019), temperate grasslands (Streeter et al. 2000; Bardgett et al. 2003), as well as boreal, temperate, and tropical forests (Nordin et al. 2001; Finzi and Berthrong 2005; Andersen and Turner 2013). However, it is generally accepted that oN acquisition is most prevalent—and indeed necessary for plant N budgets—in soils replete in oN and not iN (Weigelt et al. 2003; Jones et al. 2005b). This is especially relevant in cold ecosystems, where bioavailable soil N is regulated by low temperatures limiting extracellular enzyme activity and microbial mineralisation, effectively resulting in higher bioavailable oN than iN concentrations (Kielland 1994, 2001; Atkin 1996). Indeed, iN availability in Arctic and sub-Arctic ecosystems is insufficient to meet plant requirements without the direct uptake of oN (Kielland 2001; Lipson and Näsholm 2001; Näsholm et al. 2009). Therefore, investigations into cold systems that only considered plant iN uptake may have greatly underestimated plant-available N. This will have important implications for describing and quantifying plant-soil-N fluxes.
Despite a wealth of literature on plant oN uptake and its importance for N budgets in northern high latitudes, oN acquisition by sub-Antarctic flora has been largely overlooked, (with one exception; Schmidt and Stewart 1999). Furthermore, most sub-Antarctic nutrient- and/or N-cycling descriptions (e.g., Bergstrom and Chown 1999; Pendlebury and Barnes-Keoghan 2007; Selkirk 2007) pivots on work that assumed plant-available N was limited to iN, without accounting for potential oN acquisition (Smith and Steenkamp 1992a). The vegetated lowland (below 300 m a.s.l) soils of sub-Antarctic islands, such as Marion Island (− 46.90°, 37.75°), are typically highly organic (Smith 1978a). For example, soil oN on Marion Island makes up ca. 99.9% of total soil N (20 mg g−1 oN and < 0.1 mg g−1 iN, Table S1) if uninfluenced by large vertebrates such as seabirds and pinnipeds (Smith 1978b). Furthermore, plant N-requirements (158 mg N m−2 d−1; Smith 1988) of a Marion Island mire-grassland community during the growing season are not met by the low iN mineralisation rates (net mineralisation 48 mg N m−2 d−1) (Smith and Steenkamp 1992a). These authors assumed that only iN was relevant for plant nutrition and iN excretion by soil macrofauna was assumed to account for this discrepancy (Smith and Steenkamp 1992b, c, 1993; Smith 2008). Measurements of dissolved organic N (DON), which more accurately represent bioavailable N at a given time, show higher concentrations of DON than iN at sites with lower vertebrate influence (e.g., 82% of total dissolved N, TDN at a coastal site), and similar DON and iN concentrations when proximal to vertebrates (e.g., 58% of TDN is oN; Table S2). However, to date there has been no investigation into whether plants in this ecosystem are able to access the large pool of oN.
In addition to the important role oN acquisition may play in plant nutrition, there is evidence that oN relative to iN uptake affects plant growth and allocation. Compared to iN, the simultaneous uptake of C with N provides a net assimilation advantage to oN acquisition, the so-called “C-bonus” (Zerihun et al. 1998; Franklin et al. 2017). For example, oN (6 mM glutamine) relative to equimolar iN (NO3− and NH4NO3) increases Arabidopsis thaliana relative growth rates and root fractions, due to higher N use efficiency (NUE) and the low diffusibility of oN compounds in soil, respectively (Cambui et al. 2011; Franklin et al. 2017). However, plant responses to N-forms differ, as studies have also shown decreased root investment with amino acid application (Walch-Liu et al. 2006; Miller et al. 2007). These differences may be species-specific or depend on the concentration of the respective N-forms provided (Walch-Liu et al. 2006; Lonhienne et al. 2014). However, should N-form affect plant nutrition and allocation in the natural environment, the N-form predominantly acquired may have important ecological implications. While the effects of N-form and N concentration have been widely studied, most of this literature focusses on agricultural species (e.g., Padgett and Leonard 1996; Jämtgård et al. 2008; Tian et al. 2008; Hassan et al. 2020) or the model plant A. thaliana (Cambui et al. 2011; Lonhienne et al. 2014; Kiba and Krapp 2016). With less literature investigating how N-forms or N concentration affect wild plant growth, little is known of the ecological significance of oN or iN uptake, such as its effects on plant growth traits, competition, or co-existence in situ.
This study investigated oN uptake and the effects of oN vs iN provision on plant growth in four Marion Island graminoid species, Polypogon magellanicus (Lam.) Finot (previously Agrostis magellanica), Poa cookii (Hook.f.) Hook.f., Agrostis stolonifera L. and Poa annua L. We hypothesised that Marion Island grasses have the capacity for oN uptake, that oN influences RGR and biomass partitioning, and that the grasses acquire oN in situ, predicting that oN acquisition would be highest in soils with low faunal influence and low iN. The capacity for potted and in situ oN and iN uptake was determined by providing plants with 15N labelled oN (glycine) or iN (15NO3− and 15NH4+). The effects of oN (glycine) and iN (NO3−) on plant growth and biomass partitioning were explored in a hydroponics experiment.
Materials and methods
Study site and species
Sub-Antarctic terrestrial biotas are confined to several small, volcanic islands in the Southern Ocean (Selkirk 2007). The islands are characterised by a hyperoceanic climate, with limited seasonal range of cool temperatures. Sub-Antarctic Marion Island experiences a mean annual temperature of 6 °C, with a ca. 4 °C seasonal range between mean winter and summer temperatures (le Roux 2008). Soils near the coast and up to ca. 300 m a.s.l. are highly organic as they hold large amounts of plant material at various stages of the decomposition process. The rates of microbial decomposition of this material is slow, limited by a combination of the low temperatures and excessive moisture contents of the soils (Smith 1987). In comparison to the reported net iN mineralisation rates of 48 mg m−2 d−1 (Smith and Steenkamp 1992a), iN mineralisation rates in another cold system (an Arctic sedge meadow) is 123.4 mg m−2 d−1 (Chapin 1996). Furthermore, and assuming a root depth of 30 cm, Marion Island mineralisation rates are 0.26 mg kg−1 d−1, which is near the lowest rates reported in a global synthesis from Risch et al. (2019). Decomposition and iN release by invertebrates is limited due to current high predation by the invasive house mouse (Smith 2002; Smith et al. 2002; McClelland et al. 2018).
The four grasses investigated included two native species, P. magellanicus and P. cookii, and two invasive (anthropogenically introduced) species, P. annua and A. stolonifera. Poa cookii is a tussock-forming, highly nitrophilous species occurring in areas influenced by vertebrates (such as seabirds and pinnipeds) (Smith 1976a). Polypogon magellanicus is a tussock-forming species with a widespread distribution across Marion Island, as a common coastal and mire species but also found inland growing epiphytically on Azorella selago cushions (Smith et al. 2001). Agrostis stolonifera is a stoloniferous, invasive species that was first recorded in 1965 and has since had major impact on the island, having spread along the coastline and where there has been human activity (le Roux et al. 2013; Greve et al. 2017). It is native to parts of Europe, Asia, and north Africa, but invasive on all other continents except for Antarctica (Govaerts et al. 2021). Poa annua, another stoloniferous and invasive species, was first recorded on Marion Island in 1948 but was probably introduced by sealers in the 1800s and has also spread along the coastline (le Roux et al. 2013; Greve et al. 2017). It is native to parts of Africa, Europe, and Asia, but is invasive on all other continents including Antarctica (Molina-Montenegro et al. 2019; Govaerts et al. 2021). All four grasses co-occur along sections of the Marion Island coastline.
Two hydroponics experiments were conducted. In the first hydroponics experiment, grasses were each grown in 1 L of water that was collected from a large mire on the east coast of the island (− 46.876°, 37.857°). Mires cover ca. 50% of the island below 300 m a.s.l. and thus contribute to a substantial fraction of the island’s annual productivity (Smith 1988; Smith et al. 2001). The experiment was conducted outdoors under natural light and temperature near the main base during March 2020. The second hydroponics experiment was conducted in South Africa at the University of Cape Town. The grasses were collected from the same site as the former experiment, and grown in 1 L Long Ashton (LA) nutrient solution (Hewitt 1966).
The field experiment investigated in situ uptake of glycine, NO3−, and NH4+ at three sites (Table S) on the east coast of Marion Island. The sites had varying degrees of vertebrate influence (thus allochthonous iN input) and were characterised according to the descriptions in Smith et al. (2001) as ‘Biotic’ for the site with high animal influence near the beach, ‘Slope’ for the site along a fern slope-complex on black lava, and ‘Fellfield’ for the site on a grey-lava ridge (Fig. 1; Table S4). All four species were present within 30 m of each other at the ‘Biotic’ site, which had high vertebrate influence by seabirds such as Diomedea exulans (Wandering Albatross); Aptenodytes patagonicus (King Penguins), and Eudyptes chrysocome (Southern Rock Hopper Penguins) and pinnipeds Mirounga leonina (Southern Elephant Seals) and Arctocephalus gazella (Antarctic Fur Seals). This site was mostly covered by the four grasses, bryophytes, and the herb Leptinella plumosa. The ‘Slope’ site, with only P. cookii present, had some inactive Procellariidae (petrels and prions) burrows, representing a site with intermediate vertebrate influence (Smith 1976a). The slope was dominated by the fern, Austroblechnum penna-marina, with some P. cookii tussocks. The ‘Fellfield’ site had no visible vertebrate influence, although it may have been frequented by mice. This site was sparsely vegetated, with some Azorella selago. cushions, Andreaea spp. and Ditrichum spp. cushion bryophytes, P. magellanicus and P. annua individuals. Seabirds and pinnipeds fertilise the soil with both oN and iN compounds (Wu et al. 2023). The soil iN, broken down from uric acid and urea, are dominated by NH4+, which is either washed out to sea, undergoes nitrification, or volatilised to NH3 and carried away by wind (Smith 1978b; Erskine et al. 1998; Wu et al. 2023).
Map of sub-Antarctic Marion Island. The white point shows the research base on the east coast, and the red points show the locations where 15N enriched oN and iN were provided to the four grasses. The points near the research base are for Polypogon magellanicus, Poa cookii, Poa annua and Agrostis stolonifera and the points further inland near the base of a scoria cone (known on the island as “Junior’s Kop”) are for Polypogon magellanicus and Poa annua on a grey-lava outcrop and Poa cookii on a slope ca. 150 m from the grey-lava
Hydroponics experiments: uptake of and plant growth on organic and inorganic N
To determine whether sub-Antarctic grasses have the capacity for oN uptake, mature, non-flowering tillers of each species (n = 12) were grown for five weeks at the end of the growing season (March–April 2020). Each replicate was grown in 1 L mire water (see Table S5) with 6 mM NaNO3, with water change and nutrient replenishments every two weeks. At five weeks, plants (n = 6 of each species) were supplied with either 6 mM 15N-glycine (98% 15N, Cambridge Isotope Laboratories, Andover, MA, USA) or 15N-NaNO3 (98% 15N, Cambridge Isotope Laboratories). The 15N solutions consisted of 20 mL 10.5 µM glycine and 20 mL 10.5 µM NO3−, one of which was isotopically distinct (i.e., contained 15N). One leaf was harvested at 12 h, and the remainder of the plant harvested at 24 h following 15N supply. Unenriched (natural abundance) leaf δ15N values were obtained from plants in a separate experiment with the same growing medium.
The LA hydroponics experiment investigated plant growth, biomass partitioning, and N-uptake rates on different concentrations (4 mM or 0.4 mM) and forms of N (glycine or NO3−). Plants (n = 6 of each species) were grown in LA solution (4 mM CaCl2, 2 mM K2SO4, 1.1 mM MgSO4, 0.14 mM H3BO3, 90 µM Fe-EDTA, 20.8 µM MnSO4, 3.3 µM CuSO4, 2.3 µM ZnSO4, 0.25 µM Na2MoO4, and a phosphate buffer (pH 5.5) of 1.87 mM NaH2PO4 and 0.13 mM Na2HPO4), modified from Hewitt (1966). This experiment was run in a controlled growth chamber with a 12/12 light–dark cycle, and PAR ca. 250 µmol m−2 s−1, at 10 °C. A pilot experiment showed that 4 mM glycine in LA solution formed a precipitate within 12 h due to changes in solution pH. To avoid this, an asynchronous design was adopted, where plants were transferred between 1 L LA solution and 1 L 2 mM CaCl2 + 4/0.4 mM-N every 24 h, maximising time for nutrient acquisition but minimising movement and thus root damage. Every eight days, nutrients were replenished, and fresh biomass weighed to determine plant RGR according to the methods described by Hoffmann and Poorter (2002). At 40 d, plants were left in their respective N treatments under constant light and 1 mL solution was sampled every 12 h for 48 h, to determine N-uptake rates. To account for water loss through evapotranspiration, initial and final solution volumes were incorporated into uptake rate calculations. Plants were then harvested, dried, and weighed.
The second fully expanded leaf from the apical meristem from each plant was used for δ15N (‰) and leaf N (%) analyses. Harvested material was dried for 48 h at 70 °C, and the dry weights used to calculate root:shoot ratios. The main tiller of two replicates of P. cookii in the 4 mM NO3− treatment died during the experiment, which caused excessive variation in root:shoot ratio due to the low mass of the emerging tillers, and thus these two replicates were excluded from root:shoot ratio analysis.
Organic and inorganic N uptake in situ
To determine whether plants access oN in situ and whether this was influenced by nutrient inputs by vertebrate influence, plants were provided with 15N-glycine, 15N–NO3−, or 15N–NH4+ (15NH4Cl, 98% 15N, Cambridge Isotope Laboratories, Andover, Ma, USA). One leaf (n = 10 of each species at each site) was sampled 24 h before enrichment to determine unenriched δ15N (i.e., natural δ15N abundances). 15N treatments were added to plants (n = 10) at each site between 8 and 10 am, and one leaf harvested 24 h later. Each labelling solution (60 mL) comprised 20 mL 10 µM glycine, 20 mL 10 µM NO3− and 20 mL 10 µM NH4+, one of which was isotopically distinct (i.e., contained 15N). All leaves harvested for δ15N analyses were dried for 48 h at 70 °C and stored at room temperature until δ15N analyses.
Colorimetric assays of glycine and NO3 −
Colorimetric assays were used to determine glycine and NO3− concentrations in the LA growth experiment nutrient solution, to calculate N-uptake rates. NO3− was measured using the method described by Doane and Horwáth (2003), where VCl3 reduces NO3− to NO2−, which is captured by Griess reagents (N-1-naphthylethylenediamine dihydrochloride and sulphanilamide). Glycine was measured according to Moore and Stein (1954), where NH2-containing groups are reacted with a ninhydrin reagent. In samples without glycine addition, there were negligible amounts of NH2-containing groups, and therefore no correction was carried out for the ninhydrin protocol. Absorbance of colorimetric reactions was measured with a ThermoSpectronic spectrophotometer (Helios Epsilon model, Thermo Scientific, USA) at 540 and 570 nm for NO3− and glycine, respectively.
Leaf δ15N and leaf N analyses
For δ15N and leaf N analyses, plant leaves were ground to a fine powder using a ball mill (MM200, Retsch, Germany), and 2 mg of each sample weighed into tin capsules. The tin capsules were combusted in a Flash 2000 organic elemental analyser (Thermo Scientific, Germany), and the gasses passed into a DELTA V Plus IRMS (isotope ratio mass spectrometer) via a ConFlo IV gas control unit. Results were calibrated according to in-house standards.
Statistical analyses
All statistical analyses were performed using R statistical software, version R.4.1.1 (R Core Team 2021). Data were analysed using an Analysis of Variance (ANOVA), testing for interacting effects between species and the respective fixed effects in each experiment (i.e., plant tissue or site and N-form in the 15N enrichment experiments; N-form and -concentration for the LA growth experiment) on plant δ15N and growth variables. All residuals were checked (by visually assessing residual distributions) to conform to model assumptions, and where they did not, the data were log-transformed to meet normality and homoscedasticity assumptions. For root and shoot δ15N, log transformations did not improve homoscedasticity, and so a model was fit with the generalised least-squares (GLS) method (Zuur et al. 2009) in the ‘nlme’ package (Pinheiro and Bates 2000). Where analyses revealed significant effects (p < 0.05), Tukey Honest Significant Differences post-hoc tests were performed in the ‘emmeans’ package (Lenth 2023). For the LA and field experiments, interactions in the three-way ANOVAs resulted in complicated outputs. Because these results indicated species-specific responses to the treatments, a separate ANOVA was used for each species separately to aid interpretation. To determine the specific uptake rates over 48 h in the LA growth experiment, linear models were fitted to the solution N concentration (glycine or NO3−) over time. Uptake rates were considered detectable if there was a significant relationship (p < 0.05) between time and N concentration, and the slopes from these models used to calculate uptake rates (nmol g−1 plant biomass s−1). Uptake rates were then analysed with an ANOVA with N-treatment as factor. Where further investigations into a-priori hypotheses were relevant (i.e., for iN and oN uptake rates across all species in the LA experiment), contrasts were performed using the ‘emmeans’ package. All ANOVAs were performed in the ‘car’ package (Fox and Weisberg 2019).
Results
Hydroponics experiments: uptake of and growth on organic and inorganic N
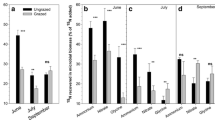
All four grasses showed significant enrichment with both 15N-glycine and 15N–NO3− when it was supplied in hydroponics (Fig. 2). Leaf material (harvested at 12 h; Fig. 2a) of A. stolonifera and P. annua had significant 15N enrichment with both N-forms, and significantly higher glycine-than NO3−-enrichment. Neither P. magellanicus nor P. cookii showed significant enrichment in the leaf material. Overall, when compared to the unenriched leaf δ15N (9 ± 0.4‰ mean ± SE), root and shoot δ15N increased substantially (mean δ15N > 100‰ for glycine and NO3−) following 15N provision (Fig. 2a, b). Furthermore, root δ15N was significantly higher following glycine than NO3− provision for all species except P. cookii (Fig. 2b). There was a significant effect of species, N-form, and plant tissue in root and shoot δ15N (p < 0.05). The best fit gls model (with the lowest AIC value) accounted for the variance structure of all three explanatory variables.
δ15N of the four study species (Polypogon magellanicus, Poa cookii, Agrostis stolonifera and Poa annua) supplied with 15N-glycine or 15N-NO3−. a δ15N 12 h after 15N enrichment in either unenriched leaves, or leaves supplied with 15N-glycine or 15N-NO3−. There was a significant interaction between species and N-treatment (F6, 47 = 53.04, p < 0.0001). Points are included to indicate the replication. b δ15N 24 h after 15N enrichment with either 15N-glycine or 15N-NO3−. There was a significant interaction between species, plant tissue, and N-form (F3, 79 = 13.76, p < 0.0001). Data are plotted on a log scale to aid visual interpretation. Letters indicate significant differences between treatment and species from an emmeans post-hoc test at the α = 0.05 significance level. Green boxplots show the shoot and leaf δ15N, and brown boxplots the root δ15N
In the LA growth experiment, there was no significant interaction between treatments and species for plant RGR. However, RGR was significantly higher in the NO3− than glycine treatment, and P. annua had significantly higher RGR than the other three species (Fig. 3a). Only P. annua resulted in a detectable decrease in N concentration in the nutrient solutions of the 4 mM-N treatments (p < 0.05; Fig. S1), and so uptake rates were analysed and compared across species in the 0.4 mM treatments only. There was a significant interaction between species and N-treatment (p < 0.05; Fig. 3b) for N-uptake rates, with higher glycine than NO3− uptake rates by A. stolonifera. Glycine uptake (nmol g−1 plant biomass s−1) was higher than the uptake of NO3− across all species (contrast: t = 3.28, p = 0.0022; Fig. 3b).
Growth in the four N treatments and uptake rates in the two 0.4 mM treatments for all study species (“Pm” is Polypogon magellanicus, “Pc” is Poa cookii, “As” is Agrostis stolonifera, and “Pa” is Poa annua) in the LA experiment. a Relative growth rate (RGR), where there was a significant difference in RGR between the N-form (F1, 90 = 5.87, p = 0.0174; left panel) and between the four species (F3, 90 = 80.58, p < 0.0001; right panel), but no difference between N concentration (middle panel). The high overlap between the boxes in the left panel is due to the interspecific variation in the different species’ RGR. b N-uptake rate (nmol g−1 s−1), where there was a significant interaction between N-form (NO3− or glycine) and species (F3, 40 = 2.92, p = 0.0456). Letters indicate significant differences between treatments and species, from an emmeans post-hoc test at the α = 0.05 significance level
There was a significant interaction between treatments and species in the LA growth experiment for root:shoot ratios and leaf N (Fig. 4), showing species-specific responses to N-form (root:shoot: F3, 78 = 5.86, p = 0.0011) and N concentration (root:shoot: F3, 78 = 4.62, p = 0.0050; leaf N: F3, 79 = 27.0, p < 0.0001). When each species was analysed separately, there was evidence that N-form (glycine relative to NO3−) had a strong effect on P. magellanicus irrespective of (total) N concentration (4 mM relative to 0.4 mM). Here, root:shoot ratios were significantly lower on glycine than NO3−. By contrast, N concentration had a strong effect on P. annua root:shoot ratio and leaf N irrespective of N-form, with high root proliferation and low leaf N at 0.4 mM relative to low root growth and high leaf N at 4 mM (Fig. 4). Both N concentration and N-form had an interactive effect on P. cookii root:shoot ratio and leaf N, which were both highest at 4 mM NO3− but lowest at 4 mM glycine. While neither N concentration nor N-form affected A. stolonifera root:shoot ratio, they had an interactive effect on leaf N, which increased with N concentration but significantly more so with NO3− than glycine (Fig. 4a, b).
Root:shoot ratio and leaf N for Polypogon magellanicus, Poa cookii, Agrostis stolonifera, and Poa annua the study species in the four N treatments in the LA growth experiment. a Root:shoot ratio, with a significant effect of N-form for P. magellanicus (F1, 21 = 9.72, p = 0.0052) but no effect of N concentration; a significant effect of N concentration for P. annua (F1, 21 = 46.60, p < 0.0001) but no effect of N-form; and a significant interaction between N concentration and N-form for P. cookii (F1, 18 = 5.38, p = 0.0323). b Leaf N, where there was a significant interaction between N concentration and N-form for P. magellanicus (F1, 20 = 14.00, p = 0.0013), A. stolonifera (F1, 19 = 19.56, p = 0.0003) and P. cookii (F1, 20 = 18.09, p = 0.0004); and a significant effect of N concentration for P. annua (F1, 21 = 67.29, p < 0.0001) but no effect of N-form. Letters indicate significant differences from an emmeans post-hoc test at the α = 0.05 significance level
Organic and inorganic N uptake in situ
The grasses showed significant δ15N enrichment relative to controls in the field sites indicating uptake of the 15N supplied. However, enrichment following oN or iN provision differed between species and sites, as shown by a significant interaction between these factors. There was significant δ15N enrichment following glycine provision at the ‘Biotic’ (A. stolonifera) and ‘Fellfield’ (P. magellanicus and P. annua) sites but not the ‘Slope’ site (Fig. 5). By contrast, δ15N enrichment following iN provision (NH4+, NO3− or both) was documented at all sites for all species (Fig. 5). For the species present at multiple sites, site significantly affected δ15N enrichment with the various N-forms. While there was only evidence for δ15N enrichment in NO3−-provided P. magellanicus and P. annua at the ‘Biotic’ site, both species showed significant δ15N enrichment in glycine- and iN-provided plants in the ‘Fellfield’ (Fig. 5). Agrostis stolonifera, which was only present at the biotic site, had significant enrichment with all 15N treatments. Poa cookii, on the other hand, only showed δ15N enrichment with iN at both the ‘Biotic’ site (NO3−) and ‘Slope’ site (both iN forms) (Fig. 5).
Leaf δ15N in the grasses at three sites in situ, for unenriched plants and plants supplied with 15N-glycine, 15N-NH4+, or 15N-NO3−. The ‘Biotic’ site is rich in soil N and proximal to vertebrate influence, the ‘Slope’ near inactive burrowing petrel burrows and thus with intermediate N concentrations, and the ‘Fellfield’ site uninfluenced by vertebrates and thus the least rich in N. There was evidence for a significant interaction between species, site, and N-form (F3, 251 = 11.32, p < 0.0001), thus separate models were run for each species. There was a significant effect of N-form on Agrostis stolonifera δ15N (F3, 36 = 9.80, p < 0.0001), and a significant interaction between site and N-form for Poa cookii (F3, 72 = 3.31, p = 0.0248), Polypogon magellanicus (F3, 71 = 7.99, p = 0.0001) and Poa annua (F3, 72 = 19.14, p < 0.0001) δ15N. Data were plotted on a log scale to aid visual interpretation. Letters show significant differences between N-forms and sites for each species from an emmeans post-hoc test at the α = 0.05 significance level
Discussion
Sub-Antarctic grasses acquire oN, which is consistent with the literature showing that plants generally have the physiological capacity for oN uptake (Näsholm et al. 2009; Paungfoo-Lonhienne et al. 2012). Indeed, glycine uptake rates surpassed those of NO3−, both when measured over time and following 15N provision. This indicates that the grasses have a high capacity for oN acquisition and readily acquire it when available at high concentrations. Considering the high oN concentrations and low mineralisation rates of Marion Island soils (Smith 1978b; Smith and Steenkamp 1992a), this suggests that direct oN acquisition plays an important, but overlooked, role in sub-Antarctic plant N nutrition.
The current study shows that sub-Antarctic grasses acquire oN in the field, particularly in sites with low iN. Plant oN use generally increases along gradients of oN availability (Schimel and Bennett 2004; Jones et al. 2005b; Moe 2013). We showed higher δ15N enrichment in glycine-provided plants at the inland site, with lower vertebrate influence and thus soil iN concentrations compared to the coastal site where high densities of seals and seabirds provide high N enrichment. Furthermore, most grasses showed significant enrichment following iN but not oN provision at sites proximal to vertebrate activity with high soil iN. Allochthonous N input by vertebrates strongly increases soil iN concentrations directly and indirectly due to the increased microbial abundance and activity associated with them (Smith 1978b, 2003; French and Smith 1986; Grobler et al. 1987). Vertebrate influence and thus high soil iN availability on the island are, however, highly localized, with vertebrate colonies largely restricted to coastal areas (Smith 1978b). For example, iN forms 0.1 – 0.03% of soil N in mires (Smith 1976b), 0.1% of soil N on black lava slopes without Procellaridae burrows (Smith 1976a), and 0.01% of grey-lava soil N (Smith et al. 2001).
Due to the use of only singly labelled isotopes (15N), intact acquisition of oN cannot be established due to the possibility of oN mineralisation prior to 15N uptake (Persson and Näsholm 2001; Jones et al. 2005a; Warren 2012). Other studies attempt to circumvent this issue with dual-labelled 13C15N, where positive regressions between the two isotopes show intact oN acquisition (Persson and Näsholm 2001; Jones et al. 2005a; Warren 2012). However, this method does not remove the chances of oN transformations: 13C and 15N may be taken up separately, or 13C may be respired immediately following uptake; circumstances that may both result in misinterpretation of the N-form utilised (Warren 2012). In addition to the field 15N data, we show oN uptake in the hydroponics experiments, where microbial activity is negligible. Other studies also show intact oN uptake in hydroponics experiments (e.g., Arkoun et al. 2012; Xiaochuang et al. 2015; Mohammadipour and Souri 2019). We therefore conclude that at least a component of the uptake is intact glycine. In the field, the low and often insufficient rates of iN mineralisation (Smith and Steenkamp 1992a) suggests that intact oN is acquired in situ, but this requires further investigation. To this end, Kranabetter et al. (2007) show that DON and iN, as opposed to only iN, are better correlated with vegetation stand height and forest N-requirements, thus DON is important for plant nutrition. We suggest that the gradients of iN and oN bioavailability, e.g., high iN near vertebrate colonies but high oN further inland, reflect the N-form predominantly acquired by sub-Antarctic plants.
Direct oN uptake by plants has energetic benefits due to the simultaneous acquisition of C with N and can affect plant growth (Franklin et al. 2017). However, contrary to other literature showing increased RGR and root allocation with oN (e.g., Cambui et al. 2011; Franklin et al. 2017), we found decreased RGR on oN and low root:shoot ratios with high (4 mM) oN supply. Biomass allocation responses to different N-forms may be concentration- and compound-specific. For example, root inhibition and stimulation of A. thaliana has been documented, but depends on the amino acid concentration and type supplied, as well as the specific eco-type under investigation (Walch-Liu et al. 2006; Lonhienne et al. 2014). Here, we detected species-specific responses in root:shoot ratio and leaf N, where P. cookii leaf N increased with root fraction but P. annua leaf N decreased with root fraction. The other species showed significant responses to the treatments, where A. stolonifera increased leaf N with high N concentrations, and P. magellanicus showed higher root growth with NO3− relative to glycine. This suggests a greater complexity to growth responses under different N-forms than that presented by Franklin et al. (2017), varying between species, concentrations, and N compounds. Plant growth strategies and their growing environment likely play a more important role in growth and allocation responses.
The different species responses to N-form and N concentration may reflect different life history strategies and thus the different environments they inhabit. For example, root proliferation under low N (irrespective of the N-form), as shown with P. annua, suggests nutrient foraging in otherwise limiting conditions (Miller and Cramer 2004). Poa annua is a ruderal alien with a near global distribution (Govaerts et al. 2021). In the Antarctic, its invasion success has been linked to high plasticity in photosynthetic traits and biomass allocation, and strong competitive ability for nutrient acquisition compared to native species (Molina-Montenegro et al. 2016; Cavieres et al. 2018; Rudak et al. 2019; Ripley et al. 2020). Contrasting P. annua biomass allocation, P. magellanicus root allocation was strongly affected by N-form irrespective of total N concentration. The combination of treatments influenced allocation and leaf N for P. cookii, with high leaf N and root fractions at 4 mM NO3− but low leaf N and root fractions at 4 mM glycine. Root proliferation under high N concentrations is a competitive strategy for N capture (Robinson et al. 1999; Hodge 2009). In an environment rich in oN with sporadic faunal iN input, responses to N-forms may confer a competitive advantage, especially considering that iN led to higher RGR than oN. These different growth strategies may influence species interactions and co-existence in the field, such as resource competition. For example, high N acquisition and biomass allocation under low N by P. annua may result in competition with native flora. The use of different N-forms and how they affect plant growth in natural settings thus represents an important avenue for future research.
Most work on Marion Island terrestrial N (and nutrient) cycling dates to the late twentieth century, resulting in a paucity of contemporary data under current paradigms and global change. It is important to quantify the extent to which oN acquisition meets plant N budgets to fully understand soil–plant-N fluxes and terrestrial energy flow on Marion Island under anthropogenic driven change such as climate warming and terrestrial invasions. Rates of biological invasions in the sub-Antarctic are high (le Roux et al. 2013; Greve et al. 2017) and may have cascading influences on the system. For example, the predation of soil invertebrates by the invasive house mouse limits nutrient cycling (Smith 2002; McClelland et al. 2018). Moreover, P. annua shows a high capacity for N acquisition, which may contribute to further expansions of this species. Understanding N sources utilised by plants is also important given the evidence for current warming in the region (le Roux and McGeoch 2008a). Soil warming may alter the relative availability of oN and iN and thus the N-form predominantly acquired by plants; for example, Kuster et al. (2016) show warming increasing soil iN availability and thus plant iN uptake. This may have important implications for vegetation N and C demands due to the differences in plant NUE between these N-forms (Franklin et al. 2017) and the different growth responses shown here. Alterations in N-form availability may also result in species composition changes, should certain species compete better for different N-forms (Pang et al. 2019). For example, warming in the maritime Antarctic and subsequent increases in moss decomposition allows the vascular plants to directly access oN in the form of proteins and peptides (Hill et al. 2019). Higher oN availability, and the mycorrhizal symbionts involved in oN uptake, is thought to facilitate the recent replacement of moss communities by vascular plants (Hill et al. 2019). Marion Island invasive flora have shown recent range expansions under climate warming (le Roux and McGeoch 2008b; le Roux et al. 2013). The role of N concentration and N-form availability in plant range expansions warrant further investigation, particularly following evidence for topographic and edaphic limitations to range expansions on Marion Island (Cramer et al. 2022).
References
Acuña-Rodríguez IS, Galán A, Torres-Díaz C, Atala C, Molina-Montenegro MA (2020) Fungal symbionts enhance N-uptake for Antarctic plants even in non-N limited soils. Front Microbiol 11:1–10. https://doi.org/10.3389/fmicb.2020.575563
Andersen KM, Turner BL (2013) Preferences or plasticity in nitrogen acquisition by understorey palms in a tropical montane forest. J Ecol 101:819–825. https://doi.org/10.1111/1365-2745.12070
Arkoun M, Sarda X, Jannin L, Laȋné P, Etienne P, Garcia-Mina J, Yvin J, Ourry A (2012) Hydroponics versus field lysimeter studies of urea, ammonium, and nitrate uptake by oilseed rape (Brassica napus L.). J Exp Bot 63(14):5245–5258. https://doi.org/10.1093/jxb/ers183
Atkin OK (1996) Reassessing the nitrogen relations of Arctic plants: a mini-review. Plant Cell Environ 19(695):704
Bardgett RD, Streeter TC, Bol R (2003) Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 84:1277–1287
Bergstrom DM, Chown SL (1999) Life at the front: history, ecology and change on southern ocean islands. Trends Ecol Evol 14:472–477. https://doi.org/10.1016/S0169-5347(99)01688-2
Cambui CA, Svennerstam H, Gruffman L et al (2011) Patterns of plant biomass partitioning depend on nitrogen source. PLoS ONE 6:1–7
Cavieres LA, Sanhueza AK, Torres-Mellado G, Casanova-Katny A (2018) Competition between native Antarctic vascular plants and invasive Poa annua changes with temperature and soil nitrogen availability. Biol Invasions 20:1597–1610. https://doi.org/10.1007/s10530-017-1650-7
Chapin DM (1996) Nitrogen mineralisation, nitrification, and denitrification in a high Arctic lowland ecosystem, Devon Island, N.W.T., Canada. Arct Alp Res 28(1):85–92. https://doi.org/10.1080/00040851.1996.12003151
Cramer MD, Hedding DW, Greve M, Midgley GF, Ripley BS (2022) Plant specialisation may limit climate-induced vegetation change to within topographic and edaphic niches on a sub-Antarctic island. Funct Ecol 36(10):2636–2648. https://doi.org/10.1111/1365-2435.14123
Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722. https://doi.org/10.1081/AL-120024647
Erskine PD, Bergstrom DM, Schmidt S et al (1998) Subantarctic Macquarie Island—a model ecosystem for studying animal-derived nitrogen sources using 15N natural abundance. Oecologia 117:187–193. https://doi.org/10.1007/s004420050647
Finzi AC, Berthrong ST (2005) The uptake of amino acids by microbes and trees in three cold-temperate forests. Ecology 86:3345–3353. https://doi.org/10.1890/04-1460
Fox J, Weisberg S (2019) An R companion to applied regression. Sage publications, Thousand Oaks
Franklin O, Cambui CA, Gruffman L et al (2017) The carbon bonus of organic nitrogen enhances nitrogen use efficiency of plants. Plant Cell Environ 40:25–35. https://doi.org/10.1111/pce.12772
French DD, Smith VR (1986) Bacterial populations in soils of a subantarctic island. Polar Biol 6:75–82. https://doi.org/10.1007/BF00258256
Govaerts R, Nic Lughadha E, Black N et al (2021) The world checklist of vascular plants, a continuously updated resource for exploring global plant diversity. Sci Data 8:1–10. https://doi.org/10.1038/s41597-021-00997-6
Greve M, Mathakutha R, Steyn C, Chown SL (2017) Terrestrial invasions on sub-Antarctic Marion and Prince Edward Islands. Bothalia Afr Biodiv Conserv 47:1–21. https://doi.org/10.4102/abc.v47i2.2143
Grobler DC, Toerien DF, Smith VR (1987) Bacterial activity in soils of a sub-Antarctic Island. Soil Biol Biochem 19:485–490. https://doi.org/10.1016/0038-0717(87)90089-7
Hassan MU, Islam MM, Wang R et al (2020) Glutamine application promotes nitrogen and biomass accumulation in the shoot of seedlings of the maize hybrid ZD958. Planta 251:1–15. https://doi.org/10.1007/s00425-020-03363-9
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition, 2nd edn. Commonwealth Agricultural Bureau, Farnham Royal
Hill PW, Broughton R, Bougoure J et al (2019) Angiosperm symbioses with non-mycorrhizal fungal partners enhance N acquisition from ancient organic matter in a warming maritime Antarctic. Ecology Letters 22:2111–2119. https://doi.org/10.1111/ele.13399
Hodge A (2009) Root decisions. Plant Cell Environ 32:628–640. https://doi.org/10.1111/j.1365-3040.2008.01891.x
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen. Trends Plant Sci 5:304–308
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42. https://doi.org/10.1093/aob/mcf140
Hutchinson HB, Miller NHJ (1912) The direct assimilation of inorganic and organic forms of nitrogen by higher plants. J Agric Sci 4:282–302. https://doi.org/10.1017/S0021859600001386
Jämtgård S, Näsholm T, Huss-Danell K (2008) Characteristics of amino acid uptake in barley. Plant Soil 302:221–231. https://doi.org/10.1007/s11104-007-9473-4
Jones DL, Healey JR, Willett VB et al (2005a) Dissolved organic nitrogen uptake by plants—an important N uptake pathway? Soil Biol Biochem 37:413–423. https://doi.org/10.1016/j.soilbio.2004.08.008
Jones DL, Shannon D, Junvee-Fortune T, Farrar JF (2005b) Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol Biochem 37:179–181. https://doi.org/10.1016/j.soilbio.2004.07.021
Kiba T, Krapp A (2016) Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol 57:707–714. https://doi.org/10.1093/pcp/pcw052
Kielland K (1994) Amino acid absorption by Arctic plants: implications for plant nutrition and nitrogen cycling. Ecology 75:2373–2383. https://doi.org/10.2307/1940891
Kielland K (2001) Short-Circuiting the nitrogen cycle: ecophysiological strategies of nitrogen uptake in plants from marginal environments. In: Ae N, Arihara J, Okada K (eds) Plant nutrient acquisition new perspective. Springer, Minato, pp 376–398
Kranabetter JM, Dawson CR, Dunn DE (2007) Indices of dissolved organic nitrogen, ammonium and nitrate across productivity gradients of boreal forests. Soil Biol Biochem 39:3147–3158. https://doi.org/10.1016/j.soilbio.2007.06.026
Kuster TM, Wilkinson A, Hill PW et al (2016) Warming alters competition for organic and inorganic nitrogen between co-existing grassland plant species. Plant Soil 406:117–129. https://doi.org/10.1007/s11104-016-2856-7
le Roux PC (2008) Climate and climate change. In: Chown SL, Froneman WP (eds) The Prince Edward Islands: land-sea interactions in a changing ecosystem. African Sun Media, Stellenbosch, pp 39–64
le Roux PC, McGeoch MA (2008a) Changes in climate extremes, variability and signature on sub-Antarctic Marion Island. Clim Change 86:309–329. https://doi.org/10.1007/s10584-007-9259-y
le Roux PC, McGeoch MA (2008b) Rapid range expansion and community reorganisation in response to warming. Glob Change Biol 14(12):2950–2962. https://doi.org/10.1111/j.1365-2486.2008.01687.x
le Roux PC, Ramaswiela T, Kalwij JM et al (2013) Human activities, propagule pressure and alien plants in the sub-Antarctic: tests of generalities and evidence in support of management. Biol Conserv 161:18–27. https://doi.org/10.1016/j.biocon.2013.02.005
Lenth RV (2023) emmeans: Estimated marginal means, aka least-squares means. R package version 1.8.4–1. https://CRAN.R-project.org/package=emmeans
Lipson DA, Monson RK (1998) Plant-microbe competition for soil amino acids in the alpine tundra: effects of freeze-thaw and dry-rewet events. Oecologia 113:406–414. https://doi.org/10.1007/s004420050393
Lipson D, Näsholm T (2001) The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia 128:305–316. https://doi.org/10.1007/s004420100693
Lonhienne TGA, Trusov Y, Young A et al (2014) Effects of externally supplied protein on root morphology and biomass allocation in Arabidopsis. Sci Rep 4:1–8. https://doi.org/10.1038/srep05055
McClelland GTW, Altwegg R, Van Aarde RJ et al (2018) Climate change leads to increasing population density and impacts of a key island invader. Ecol Appl 28:212–224. https://doi.org/10.1002/eap.1642
Miller AJ, Cramer MD (2004) Root nitrogen acquisition and assimilation. Plant Soil 274:1–36. https://doi.org/10.1007/s11104-004-0965-1
Miller AJ, Fan X, Shen Q, Smith SJ (2007) Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J Exp Bot 59:111–119. https://doi.org/10.1093/jxb/erm208
Moe LA (2013) Amino acids in the rhizosphere: from plants to microbes. Am J Bot 100:1692–1705. https://doi.org/10.3732/ajb.1300033
Mohammadipour N, Souri MK (2019) Effects of different levels of glycine in the nutrient solution on the growth, nutrient composition, and antioxidant activity of coriander (Coriandrum sativum L.). Acta Agrobot 72:1–9. https://doi.org/10.5586/aa.1759
Molina-Montenegro MA, Galleguillos C, Oses R et al (2016) Adaptive phenotypic plasticity and competitive ability deployed under a climate change scenario may promote the invasion of Poa annua in Antarctica. Biol Invasions 18:603–618. https://doi.org/10.1007/s10530-015-1033
Molina-Montenegro MA, Bergstrom DM, Chwedorzewska KJ et al (2019) Increasing impacts by Antarctica’s most widespread invasive plant species as result of direct competition with native vascular plants. NeoBiota 51:19–40. https://doi.org/10.3897/neobiota.51.37250
Moore S, Stein WH (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211:907–913. https://doi.org/10.1016/s0021-9258(18)71178-2
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48. https://doi.org/10.1111/j.1469-8137.2008.02751.x
Nordin A, Högberg P, Näsholm T (2001) Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. Oecologia 129:125–132. https://doi.org/10.1007/s004420100698
Nordin A, Schmidt I, Shaver G (2004) Nitrogen uptake by Arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 85:955–962
Padgett PE, Leonard RT (1996) Free amino acid levels and the regulation of nitrate uptake in maize cell suspension cultures. J Exp Bot 47:871–883. https://doi.org/10.1093/jxb/47.7.871
Pang Z, Jiang L, Wang S et al (2019) Differential response to warming of the uptake of nitrogen by plant species in non-degraded and degraded alpine grasslands. J Soils Sediments 19:2212–2221. https://doi.org/10.1007/s11368-019-02255-0
Paungfoo-Lonhienne C, Visser J, Lonhienne TGA, Schmidt S (2012) Past, present and future of organic nutrients. Plant Soil 359:1–18. https://doi.org/10.1007/s11104-012-1357-6
Pendlebury S, Barnes-Keoghan I (2007) Climate and climate change in the sub-Antarctic. Pap Proc Royal Soc Tasman 141:67–81. https://doi.org/10.26749/rstpp.141.1.67
Persson J, Näsholm T (2001) A GC-MS method for determination of amino acid uptake by plants. Physiol Plant 113:352–358. https://doi.org/10.1034/j.1399-3054.2001.1130308.x
Pinheiro JC, Bates DM (2000) Mixed-Effects Models in S and S-PLUS. Springer, New York. https://doi.org/10.1007/b98882
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ripley BS, Edwardes A, Rossouw MW et al (2020) Invasive grasses of sub-Antarctic Marion Island respond to increasing temperatures at the expense of chilling tolerance. Ann Bot 125:765–773. https://doi.org/10.1093/aob/mcz156
Risch AC, Zimmermann S, Ochoa-Hueso R et al (2019) Soil net nitrogen mineralisation across global grasslands. Nat Commun 1:1–10. https://doi.org/10.1038/s41467-019-12948-2
Robinson D, Hodge A, Griffiths BS, Fitter AH (1999) Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proc Royal Soc B: Biol Sci 266:431–435. https://doi.org/10.1098/rspb.1999.0656
Rudak A, Wódkiewicz M, Znój A et al (2019) Plastic biomass allocation as a trait increasing the invasiveness of annual bluegrass (Poa annua L.) in Antarctica. Polar Biol 42:149–157. https://doi.org/10.1007/s00300-018-2409-z
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schmidt S, Stewart GR (1999) Glycine metabolism by plant roots and its occurrence in Australian plant communities. Aust J Plant Physiol 26:253–264
Schmidt IK, Jonasson S, Shaver GR et al (2002) Mineralization and distribution of nutrients in plants and microbes in four arctic ecosystems: responses to warming. Plant Soil 242:93–106. https://doi.org/10.1023/A:1019642007929
Selkirk P (2007) The nature and importance of the sub-Antarctic. Pap Proc Royal Soc Tasman 141:1–6. https://doi.org/10.26749/rstpp.141.1.1
Smith VR (1976a) The effect of burrowing species of Procellariidae on the nutrient status of inland tussock grasslands on Marion Island. J South Afr Bot 42:265–272
Smith VR (1976b) Standing crop and nutrient status of Marion Island (sub-Antarctic) vegetation. Journal of South African Botany 42:231–263
Smith VR (1978a) Soil chemistry of Marion Island (Subantarctic). S Afr J Sci 74:174–175
Smith VR (1978b) Animal-plant-soil nutrient relationships on Marion Island (Subantarctic). Oecologia 32:239–253. https://doi.org/10.1007/BF00366075
Smith VR (1987) The environment and biota of Marion Island. S Afr J Sci 83:211–220
Smith VR (1988) Production and nutrient dynamics of plant communities on a sub-Antarctic Island 5. Nutrient budgest and turnover times for mire-grasslands, fjaeldmark and fernbrakes. Polar Biol 8:255–269. https://doi.org/10.1007/BF00263174
Smith VR (2002) Climate change in the sub-Antarctic: an illustration from Marion Island. Clim Change 52:345–357. https://doi.org/10.1023/A:1013718617277
Smith VR (2003) Soil respiration and its determinants on a sub-Antarctic island. Soil Biol Biochem 35:77–91. https://doi.org/10.1016/S0038-0717(02)00240-7
Smith VR (2008) Energy flow and nutrient cycling in the Marion Island terrestrial ecosystem: 30 years on. Polar Rec 44:211–226. https://doi.org/10.1017/S0032247407007218
Smith VR, Steenkamp M (1992a) Soil nitrogen transformations on a sub-Antarctic island. Antarct Sci 4:41–50. https://doi.org/10.1007/BF00317365
Smith VR, Steenkamp M (1992b) Soil macrofauna and nitrogen on a sub-Antarctic island. Oecologia 92:201–206
Smith VR, Steenkamp M (1992c) Macroinvertebrates and litter nutrient release on a sub-Antarctic Island. S Afr J Bot 58:105–116. https://doi.org/10.1016/s0254-6299(16)30880-8
Smith VR, Steenkamp M (1993) Macroinvertebrates and peat nutrient mineralization on a sub-Antarctic island. S Afr J Bot 59:106–108. https://doi.org/10.1016/s0254-6299(16)30782-7
Smith VR, Steenkamp M, Gremmen NJM (2001) Terrestrial habitats on sub-Antarctic Marion Island: their vegetation, edaphic attributes, distribution and response to climate change. S Afr J Bot 67:641–654. https://doi.org/10.1016/S0254-6299(15)31195-9
Smith VR, Avenant NL, Chown SL (2002) The diet and impact of house mice on a sub-Antarctic island. Polar Biol 25:703–715. https://doi.org/10.1007/s00300-002-0405-8
Streeter TC, Bol R, Bardgett RD (2000) Amino acids as a nitrogen source in temperate upland grasslands: the use of dual labelled ((13)C, (15)N) glycine to test for direct uptake by dominant grasses. Rapid Commun Mass Spectrom 14:1351–1355. https://doi.org/10.1002/1097-0231(20000815)14:15%3c1351::AID-RCM23%3e3.0.CO;2-9
Tian Q, Chen F, Liu J et al (2008) Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J Plant Physiol 165:942–951. https://doi.org/10.1016/j.jplph.2007.02.011
Virtanen AI, Linkola H (1946) Organic nitrogen compounds as nitrogen nutrition for higher plants. Nature 157:515
Walch-Liu P, Ivanov II, Filleur S et al (2006) Nitrogen regulation of root branching. Ann Bot 97:875–881. https://doi.org/10.1093/aob/mcj601
Warren CR (2012) Post-uptake metabolism affects quantification of amino acid uptake. New Phytol 193:522–531. https://doi.org/10.1111/j.1469-8137.2011.03933.x
Weigelt A, King R, Bol R, Bardgett RD (2003) Inter-specific variability in organic nitrogen uptake of three temperate grassland species. J Plant Nutr Soil Sci 166:606–611. https://doi.org/10.1002/jpln.200320322
Wright DE (1962) Amino acid uptake by plant roots. Arch Biochem Biophys 97:174–180. https://doi.org/10.1016/0003-9861(62)90061-9
Wu L, Sheng M, Liu X, Zheng Z, Emslie SD, Yang N, Wang X, Nie Y, Jin J, Xie Q, Chen S, Zhang D, Su S, Zhong S, Hu W, Deng J, Zhu J, Qi Y, Lie C, Fu P (2023) Molecular transformation of organic nitrogen in Antarctic penguin guano-affected soil. Environ Int 172:1–13. https://doi.org/10.1016/j.envint.2023.107796
Xiaochuang C, Lianghuan W, Ling Y, Xiaoyan L, Yuanhong Z, Qianya J (2015) Uptake and uptake kinetics of nitrate, ammonium and glycine by pakchoi seedlings (Brassica Campestris L. ssp. Chinensis L. Makino). Sci Hortic 186:247–253. https://doi.org/10.1016/j.scienta.2015.02.010
Zerihun A, McKenzie BA, Morton JD (1998) Photosynthate costs associated with the utilization of different nitrogen-forms: Influence on the carbon balance of plants and shoot-root biomass partitioning. New Phytol 138:1–11. https://doi.org/10.1046/j.1469-8137.1998.00893.x
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This study was made possible with the financial support of the South African National Research Foundation (NRF), South African National Antarctic Programme (SANAP) grant (110734). The research was conducted under DSI permit 010/2019. Stephni van der Merwe provided the field site map.
Funding
Open access funding provided by University of Cape Town.
Author information
Authors and Affiliations
Contributions
NCMP, MDC, MG, and BSR conceived and designed research. NP conducted experiments, analysed data, and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study is based on plant data and did not require ethics approval.
Informed consent
This study is based on plant data and did not require informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pallett, N.C.M., Ripley, B.S., Greve, M. et al. Rethinking the sub-Antarctic terrestrial N-cycle: evidence for organic N acquisition by Marion Island grasses. Polar Biol 47, 411–423 (2024). https://doi.org/10.1007/s00300-024-03240-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-024-03240-1