WO2005089317A2 - Immune response modifier formulations and methods - Google Patents
Immune response modifier formulations and methods Download PDFInfo
- Publication number
- WO2005089317A2 WO2005089317A2 PCT/US2005/008576 US2005008576W WO2005089317A2 WO 2005089317 A2 WO2005089317 A2 WO 2005089317A2 US 2005008576 W US2005008576 W US 2005008576W WO 2005089317 A2 WO2005089317 A2 WO 2005089317A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- alkyl
- formulation
- amines
- substituted
- Prior art date
Links
- 0 C*1(*)Cc2nc(N)c3nc(*)[n](*)c3c2C=CC1 Chemical compound C*1(*)Cc2nc(N)c3nc(*)[n](*)c3c2C=CC1 0.000 description 8
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
- A61K31/202—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids having three or more double bonds, e.g. linolenic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4375—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a six-membered ring having nitrogen as a ring heteroatom, e.g. quinolizines, naphthyridines, berberine, vincamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/107—Emulsions ; Emulsion preconcentrates; Micelles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/12—Keratolytics, e.g. wart or anti-corn preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Definitions
- Pharmaceutical formulations containing IRM compounds are disclosed in U.S. Patent Nos. 5,238,944; 5,939,090; and 6,425,776; European Patent 0 394 026; and U.S. Patent Publication 2003/0199538.
- IRM compounds include preservatives such as methylparaben, sorbic acid, propylene glycol, etc.
- the mechanism for the antiviral and antitumor activity of these IRM compounds is thought to be due in substantial part to enhancement of the immune response by induction of various important cytokines (e.g., interferons, interleukins, tumor necrosis factor, etc.).
- cytokines e.g., interferons, interleukins, tumor necrosis factor, etc.
- Such compounds have been shown to stimulate a rapid release of certain monocyte/macrophage-derived cytokines and are also capable of stimulating B cells to secrete antibodies, which play an important role in these IRM compounds' antiviral and antitumor activities.
- IFN interferon
- IL-1 Interleukin-1
- IL-6 Interleukin-6
- IRMs SUMMARY OF THE INVENTION
- factors include, e.g., irritation of the skin to which the formulation is applied; formulation wash away; insolubility and/or degradation of the IRM compound in the formulation; physical instability of the formulation (e.g., separation of components, thickening, precipitation/agglomeration of active ingredient, and the like); degradation of excipients; poor permeation; and undesired systemic delivery of the topically applied IRM compound.
- IRMs containing a 2-aminopyridine moiety fused to a five-membered nitrogen- containing heterocyclic ring interact with preservatives such as sorbic acid, resulting in decreased concentrations (relative to the initial concentrations in the freshly prepared formulation) of both the IRM and preservative, with the resulting formation of unwanted impurities.
- IRMs interact with intennediates and products resulting from autoxidation of the sorbic acid preservative. It has been discovered that stability can be improved through the addition of a compound acting as an antioxidant.
- the antioxidant may beneficially have hydrogen atom donating functionality.
- stability of the formulation maybe further improved by adding a chelating agent.
- the present invention provides a pharmaceutical formulation that includes: an immune response modifier (IRM) compound having a 2- aminopyridme moiety fused to a five-membered nitrogen-containing heterocyclic ring; a preservative system that includes sorbic acid, esters thereof, salts thereof, or combinations thereof; and an antioxidant.
- IRM immune response modifier
- a chelating agent may also beneficially be included in any of the formulations described herein.
- a fatty acid, a hydrophobic, aprotic component miscible with the fatty acid and including a hydrocarbyl group of 7 or more carbon atoms, or combinations thereof may also be included in any of the formulations described herein.
- the present invention provides a pharmaceutical formulation that includes: an immune response modifier (IRM) compound having a 2-aminopyridine moiety fused to a five-membered nitrogen- containing heterocyclic ring; a preservative system that includes a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof; an antioxidant having hydrogen atom donating functionality; a fatty acid; and a hydrophobic, aprotic component miscible with the fatty acid and having a hydrocarbyl group of 7 or more carbon atoms.
- a chelating agent may also beneficially be included.
- the present invention provides a pharmaceutical formulation that includes: 0.001% by weight to 5.0% by weight of an immune response modifier (IRM) compound having a 2-aminopyridine moiety fused to a five-membered nitrogen-containing heterocyclic ring (preferably, an imidazonaphthyridine amine, and more preferably 2-methyl-l-(2-methylpropyl)-lH-imidazo[4,5-c][l,5]naphthyridin-4- amine); a preservative system; 0.001% by weight to 0.2% by weight of an antioxidant having hydrogen atom donating functionality; 0 to 0.1% by weight of a chelating agent; 1 % by weight to 30% by weight of a fatty acid; 1 % by weight to 15% by weight of a medium-chain triglyceride; 0.2% by weight to 2.0% by weight of a viscosity enhancing agent; 0.1 % by weight to 6.0% by weight of an emulsifier; and water; wherein the formulation
- the preservative system includes: 0.02% by weight to 0.2% by weight of a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof; 0 to 10.0% by weight of a preservative enhancing solubilizer; and 0.05% by weight to 0.2% by weight of a secondary preservative compound.
- a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof
- 0 to 10.0% by weight of a preservative enhancing solubilizer and 0.05% by weight to 0.2% by weight of a secondary preservative compound.
- Certain embodiments of the present invention include a hydrophilic viscosity agent, such as those selected from cellulose ethers and carbomers. If used, the hydrophilic viscosity agent is preferably present in an amount of 0.2% by weight to 2.0% by weight.
- Other useful additives include, for example, a p ⁇ adjuster, an e
- the present invention also provides methods.
- the present invention provides a method of stabilizing a pharmaceutical formulation that includes: an immune response modifier (IRM) compound having a 2-aminopyridine moiety fused to a five-membered nitrogen- containing heterocyclic ring; and a preservative system that includes a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof.
- the method includes adding an antioxidant and optionally a chelating agent to the formulation.
- the present invention provides a method for delivering an immune response modifier (IRM) to a dermal surface. The method includes selecting a formulation of the present invention and applying the selected formulation to the dermal and/or mucosal surface.
- the present invention provides a method of treating a dermal associated condition (particularly, actinic keratosis).
- the method includes applying to a dermal surface of a patient in need thereof a pharmaceutical formulation of the present invention.
- a "sorbic acid preservative" means sorbic acid, esters of sorbic acid, salts of sorbic acid, or combinations thereof.
- “remains substantially constant” means that the concentration of sorbic acid preservative in an IRM-containing formulation does not decrease by more than 15% of the initial concentration (i.e., its concentration when initially formulated) when stored for at least 6 months at 40°C and 75% relative humidity.
- the present invention provides pharmaceutical formulations that include an immune response modifier containing a 2-aminopyridine fused to a five-membered nitrogen-containing heterocyclic ring, a preservative system that includes a sorbic acid preservative (i.e., sorbic acid, esters of sorbic acid, salts of sorbic acid, or combinations thereof).
- a sorbic acid preservative i.e., sorbic acid, esters of sorbic acid, salts of sorbic acid, or combinations thereof.
- sorbic acid preservative i.e., sorbic acid, esters of sorbic acid, salts of sorbic acid, or combinations thereof.
- such formulations are stabilized through the inco ⁇ oration of an antioxidant, more preferably an antioxidant having hydrogen atom donating functionality. Additional stability, particularly of the antioxidant, can be obtained through the inco ⁇ oration of a chelating agent.
- the concentration of the sorbic acid preservative in an IRM-containing formulation remains substantially constant relative to its initial concentration (i.e., its concentration when initially formulated) when stored for at least 6 months at 40°C and 75% relative humidity.
- “remains substantially constant” means that the concentration of sorbic acid preservative in an IRM-containing formulation does not decrease by more than 15% of the initial concentration (i.e., its concentration when initially formulated) when stored for at least 6 months at 40°C and 75% relative humidity.
- the concentration of the sorbic acid preservative in an IRM-containing formulation does not decrease by more than 10% of the initial concentration when stored for at least 6 months at 40°C and 75% relative humidity.
- formulations described herein can be in the form of an oil-in-water emulsion such as a cream or a lotion.
- an emulsion can include an oil phase that includes one or more IRM compounds, a fatty acid in an amount sufficient to solubilize the IRM compound(s), a hydrophobic, aprotic component; and an aqueous phase that includes a preservative system, and a hydrophilic viscosity enhancing agent.
- Such components, as well as all others of the formulations described herein, are preferably pharmaceutically acceptable.
- the present invention provides a formulation that includes an immune response modifier containing a 2-aminopyridine moiety fused to a five- membered nitrogen-containing heterocyclic ring.
- Immune response modifier compounds include compounds that possess potent immunomodulating activity including but not limited to antiviral and antitumor activity.
- Certain IRMs modulate the production and secretion of cytokines.
- certain IRM compounds induce the production and secretion of cytokines such as, e.g., Type I interferons, TNF- ⁇ , IL-1, IL-6, IL-8, IL-10, IL-12, MIP-1, and/or MCP-1.
- IRM compounds can inhibit production and secretion of certain T H cytokines, such as IL-4 and IL-5. Additionally, some IRM compounds are said to suppress IL-1 and TNF (U.S. Patent No. 6,518,265).
- IRM compounds suitable for use in the invention include compounds having a
- 2-aminopyridine fused to a five-membered nitrogen-containing heterocyclic ring Such compounds include, for example, imidazoquinoline amines including, but not limited to, substituted imidazoquinoline amines such as, for example, amide substituted imidazoquinoline amines, sulfonamide substituted imidazoquinoline amines, urea substituted imidazoqumolme amines, aryl ether substituted imidazoquinoline amines, heterocyclic ether substituted imidazoquinoline amines, amido ether substituted imidazoquinoline amines, sulfonamido ether substituted imidazoquinoline amines, urea substituted imidazoquinoline ethers, thioether substituted imidazoquinoline amines, and 6-, 7-, 8-, or 9-aryl or heteroaryl substituted imidazoquinoline amines; tetrahydroimidazoquinoline amines including, but not limited to,
- the IRM compound can be chosen from lH-imidazo[4,5-c]quinolin-4-amines defined by one of Formulas I-V below:
- Rn is selected from alkyl of one to ten carbon atoms, hydroxyalkyl of one to six carbon atoms, acyloxyalkyl wherein the acyloxy moiety is alkanoyloxy of two to four carbon atoms or benzoyloxy, and the alkyl moiety contains one to six carbon atoms, benzyl, (phenyl)ethyl and phenyl, said benzyl, (phenyl)ethyl or phenyl substituent being optionally substituted on the benzene ring by one or two moieties independently selected from alkyl of one to four carbon atoms, alkoxy of one to four carbon atoms and halogen, with the proviso that if said benzene ring is substituted by two of said moieties, then said moieties together contain no more than six carbon atoms; R ⁇ is selected from hydrogen, alkyl of one to eight carbon atoms, benzyl, (phenyl)e
- R 14 is -CHR x Ry wherein R y is hydrogen or a carbon-carbon bond
- R y is hydrogen or a carbon-carbon bond
- R x is alkoxy of one to four carbon atoms, hydroxyalkoxy of one to four carbon atoms, 1 -alkynyl of two to ten carbon atoms, tefrahydropyranyl, alkoxyalkyl wherein the alkoxy moiety contains one to four carbon atoms and the alkyl moiety contains one to four carbon atoms, or 2-, 3-, or 4-pyridyl
- R 24 is selected from hydrogen, alkyl of one to four carbon atoms, phenyl, and substituted phenyl wherein
- R ⁇ 5 is selected from hydrogen; straight chain or branched chain alkyl containing one to ten carbon atoms and substituted straight chain or branched chain alkyl containing one to ten carbon atoms, wherein the substituent is selected from cycloalkyl containing three to six carbon atoms and cycloalkyl containing three to six carbon atoms substituted by straight chain or branched chain alkyl containing one to four carbon atoms; straight chain or branched chain alkenyl containing two to ten carbon atoms and substituted straight chain or branched chain alkenyl containing two to ten carbon atoms, wherein the substituent is selected from cycloalkyl containing three to six carbon atoms and cycloalkyl containing three to six carbon atoms substituted by straight chain or branched chain alkyl containing one to four carbon atoms; hydroxyalkyl of one to six carbon atoms; alkoxyalkyl wherein the alkoxy moiety contains
- R T are independently selected from hydrogen, alkyl of one to four carbon atoms, phenyl, and substituted phenyl wherein the substituent is selected from alkyl of one to four carbon atoms, alkoxy of one to four carbon atoms, and halogen;
- X is selected from alkoxy containing one to four carbon atoms, alkoxyalkyl wherein the alkoxy moiety contains one to four carbon atoms and the alkyl moiety contains one to four carbon atoms, hydroxyalkyl of one to four carbon atoms, haloalkyl of one to four carbon atoms, alkylamido wherem the alkyl group contains one to four carbon atoms, amino, substituted amino wherein the substituent is alkyl or hydroxyalkyl of one to four carbon atoms, azido, chloro, hydroxy, 1-mo ⁇ holino, 1-pynolidino, alkylthio of one to four carbon atoms; and R 5 is selected from hydrogen,
- R 16 is selected from hydrogen; cyclic alkyl of three, four, or five carbon atoms; sfraight chain or branched chain alkyl containing one to ten carbon atoms and substituted sfraight chain or branched chain alkyl containing one to ten carbon atoms, wherein the substituent is selected from cycloalkyl containing three to six carbon atoms and cycloalkyl containing three to six carbon atoms substituted by straight chain or branched chain alkyl containing one to four carbon atoms; fluoro- or chloroalkyl containing from one to ten carbon atoms and one or more fluorine or chlorine atoms; straight chain or branched chain alkenyl containing two to ten carbon atoms and substituted straight chain or branched chain alkenyl containing two to ten carbon atoms, wherein the substituent is selected from cycloalkyl containing three to six carbon atoms
- (phenyl)ethyl or phenyl substituent being optionally substituted on the benzene ring by one or two moieties independently selected from alkyl of one to four carbon atoms, alkoxy of one to four carbon atoms, and halogen, with the proviso that when said benzene ring is substituted by two of said moieties, then the moieties together contain no more than six carbon atoms; and -CHR x R y wherein R y is hydrogen or a carbon-carbon bond, with the proviso that when R y is hydrogen R x is alkoxy of one to four carbon atoms ' , hydroxyalkoxy of one to four carbon atoms, 1 -alkynyl of two to ten carbon atoms, tefrahydropyranyl, alkoxyalkyl wherein the alkoxy moiety contains one to four carbon atoms and the alkyl moiety contains one to four carbon atoms, 2-, 3-, or 4-pyr
- the IRM compound can be chosen from imidazopyridine amines defined by Formula VII below:
- Z is selected from -(CH 2 ) P - wherein p is 1 to 4; -(CH2) a -C(RDRE)(CH 2 )b-, wherein a and b are integers and a+b is 0 to 3, RD is hydrogen or alkyl of one to four carbon atoms, and R E is selected from alkyl of one to four carbon atoms, hydroxy, -ORp wherein R F is alkyl of one to four carbon atoms, and
- R G and R' G are independently hydrogen or alkyl of one to four carbon atoms; and -(CH 2 ) a -(Y)-(CH 2 )b- wherein a and b are integers and a+b is 0 to 3, and Y is O, S, or -NRj- wherein Rj is hydrogen or alkyl of one to four carbon atoms; q is O or l; and R 8 is selected from alkyl of one to four carbon atoms, alkoxy of one to four carbon atoms, and halogen, and pharmaceutically acceptable salts thereof.
- the IRM compound can be chosen from thiazoloquinoline amines, oxazoloquinoline amines, thiazolopyridine amines, oxazolopyridine amines, thiazolonaphthyridine amines and oxazolonaphthyridine amines defined by Fonnula IX below:
- R ⁇ 9 is selected from oxygen, sulfur and selenium;
- R 29 is selected from -hydrogen; -alkyl; -alkyl-OH; -haloalkyl; -alkenyl; -alkyl-X-alkyl; -alkyl-X-alkenyl; -alkenyl-X-alkyl; -alkenyl-X-alkenyl; -alkyl-N(R 59 ) 2 ; -alkyl-N 3 ; -alkyl-O-C(O)-N(R 59 ) 2 ; -heterocyclyl; -alkyl-X-heterocyclyl; -alkenyl-X-heterocyclyl; -aryl; -alkyl-X-aryl;
- R 39 and R ⁇ are each independently: -hydrogen; -X-alkyl; -halo; -haloalkyl; -N(R 59 ) 2 ; or when taken together, R 39 and Rj 9 form a fused aromatic, heteroaromatic, cycloalkyl or heterocyclic ring;
- X is selected from-O-, -S- -NR 59 - -C(O)-, -C(O)O-, -OC(O)-, and a bond; and each R 59 is independently H or C ⁇ - 8 alkyl; and pharmaceutically acceptable salts thereof.
- the IRM compound can be chosen from imidazonaphthyridine amines and imidazotefrahydronaphthyridine
- Ri ⁇ o is selected from: - hydrogen; -C 1 . 20 alkyl or C 2 .
- 2C alkenyl that is unsubstituted or substituted by one or more substituents selected from: -aryl; -heteroaryl; -heterocyclyl; -O-C 1 -20 alkyl; -O-(C 1 - 20 alkyl) 0 .i-aryl; -O-(C ⁇ -2o alkyl)o- ⁇ -heteroaryl; -O-(C ⁇ - 20 alkyl) 0 - ⁇ -heterocyclyl; -CO-O-d-20 alkyl; -S(O)o-2 -C ⁇ .2o alkyl; -S(O)o.
- Y is -N- or -CR-;elected from: -hydrogen; -C ⁇ -10 alkyl;
- each R 3 ⁇ o is independently selected from hydrogen and C ⁇ - 10 alkyl; and each R is independently selected from hydrogen, C ⁇ - ⁇ 0 alkyl, C ⁇ - 10 alkoxy, halogen and trifluoromethyl;
- B is -NR-C(R) 2 -C(R) 2 -C(R) 2 -; -C(R) 2 -NR-C(R) 2 -C(R) 2 -; -C(R) 2 -C(R) 2 -NR-C(R) 2 - or -C(R) 2 -C(R) 2 -C(R) 2 -NR-;
- R 111 is selected from: - hydrogen; -Ci.
- o alkyl or C 2 -2o alkenyl that is unsubstituted or substituted by one or more substituents selected from: -aryl; -heteroaryl; -heterocyclyl; -O-C ⁇ - 20 alkyl; -O-(Ci. 2 o alkyl)o-i-aryl; -O-(C !
- alkenyl-NRsn-Q-X-Rm wherein Q is - CO- or -SO 2 -; X is a bond, -O- or -NR3 11 - and Rm is aryl; heteroaryl; heterocyclyl; or ⁇ Ci- 20 alkyl or C 2 - 2 0 alkenyl that is unsubstituted or substituted by one or more substituents selected from: -aryl; -heteroaryl; -heterocyclyl; -O-C ⁇ - 20 alkyl; -O-(C 1 .
- Y is -N- or -CR-;elected from: -hydrogen;
- the IRM compound can be chosen from 1H- imidazo[4,5-c]quinolin-4-amines and tefrahydro- lH-imidazo[4,5-c]quinolin-4-amines defined by Formulas XII, XIII and XIV below:
- R112 is -alkyl-NR ⁇ -CO-Ri ⁇ or -alkenyl-NR 3 i2-CO- R_n 2 wherein R 4 ⁇ 2 is aryl, heteroaryl, alkyl or alkenyl, each of which may be unsubstituted or substituted by one or more substituents selected from: -alkyl; -alkenyl; -alkynyl; -(alkyl)o- ⁇ -aryl; -(alkyl)o- ⁇ -(substituted aryl);
- R412 is wherein R512 is an aryl, (substituted aryl), heteroaryl, (substituted heteroaryl), heterocyclyl or (substituted heterocyclyl) group; R 212 is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -(substituted aryl); -heteroaryl; -(substituted heteroaryl); -heterocyclyl; -(substituted heterocyclyl); -alkyl-O-alkyl; -alkyl-O-alkenyl; and -alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -N(R 3 ⁇ 2 )
- R ⁇ 3 is -alkyl-NR 3 i3- SO 2 -X-R413 or -alkenyl-NR 313 - SO 2 -X-R413 ;
- X is a bond or -NR 5 ⁇ 3 -;
- R» 3 is aryl, heteroaryl, heterocyclyl, alkyl or alkenyl, each of which may be unsubstituted or substituted by one or more substituents selected from: -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -substituted cycloalkyl;
- R 2 ⁇ is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -substituted aryl; -heteroaryl; -substituted heteroaryl; - alkyl-O-alkyl; - alkyl-O- alkenyl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -CO-Ci-io alkyl; -CO-O-Ci.io alkyl; -N 3 ; -aryl; -substi
- the IRM compound can be chosen from 1H- irnidazo[4,5-c]quinolin-4-amines and tefrahydro- lH-imidazo[4,5-c]quinolin-4-amines defined by Formulas XV, XVI, XVII, XVIII, XIX, XX, XXI, XXII, XXIII, XXIV,
- X is -C ⁇ R 5 ⁇ 5 -, -CHR 5 ⁇ 5 -alkyl-, or -CHR 5 i 5 -alkenyl-;
- Rn 5 is selected from: -R 41 5-CR 31 S-Z-R615— alkyl; -lUis-CR ⁇ s-Z-Reis — alkenyl; -R 4 i5-CR 3 i 5 -Z-R 6 i5— aryl; -R-us-CR ⁇ s-Z-Reis — heteroaryl; - HS -CR 31S -Z-R 015 — heterocyclyl; -R 41 5-NR 71 5 -C 31 5-R ⁇ 1S— alkyl; -R415-NR7.5 -CR 3 i 5 -R ⁇ 5i 5 — alkenyl; -R 4 i 5 -NR 7 ⁇ 5 -CR 3 i 5 -R 6 i 5 -aryl; -R
- X is -CHR 5 ⁇ 6 -, -CHR 5 ⁇ 6 -alkyl-, or -CHR 5 ⁇ 6 -alkenyl-;
- R ⁇ i 6 is selected from: -R4 1 6-CR 3 i6-Z-R6i6 — lkyl; -R416-CR316-Z-R6I6 — aryl; - H6 -CR3 l ⁇ -Z-R ⁇ i ⁇ — heteroaryl; -R 41 6-CR316-Z-R ⁇ 16 — heterocyclyl; -R416- R716 -CR 3 i6-R6i 6 — alkyl; -R-H6-NR716 -CR316-R6I6 — alkenyl; -R 4 i 6 -NR7i6-CR3i 6 -R 6 i 6 -aryl; -R i6-NR7i6-CR3i6-R6i6-hetero
- X is -CHR 317 -, -CHR 3 i -alkyl-, or -CHR 3 i 7 -alkenyl-;
- Ri i 7 is selected from: -alkenyl; -aryl; and -R- -aryl;
- R 2 ⁇ is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkyl-Y-alkyl; -alkyl- Y- alkenyl; -alkyl- Y-aryl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -N(R 3 ⁇ 7 ) 2 ; -CO-Ci-io alkyl; -CO-O-C MO alkyl; -N 3 ; -aryl; -heteroaryl;
- X is -CHR 3 ⁇ 8 -, -CHR 3 ⁇ 8 -alkyl-, or -CHR 3 ⁇ 8 -alkenyl-;
- R ⁇ s is selected from: -aryl; -alkenyl; and -R t is-aryl;
- R 2 ⁇ 8 is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkyl-Y-alkyl; -alkyl- Y-aryl; - alkyl- Y- alkenyl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -N(R 3 ⁇ s) 2 ; -CO-N(R 3 ⁇ 8 ) 2 ; -CO-CMO alkyl; -CO-O-CMO alkyl; -N 3
- X is -CHR 319 -, -CHR 3 j 9 -alkyl-, or -CHR 3 ⁇ 9 -alkenyl-;
- Rn is selected from: -heteroaryl; -heterocyclyl; -R 4 i 9 - heteroaryl; and -Rnsr-heterocyclyl;
- R 2 ⁇ 9 is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkyl-Y-alkyl; -alkyl- Y- alkenyl; -alkyl- Y-aryl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -N(R 3 ⁇ 9 ) 2 ; -CO-N(R 3 ⁇ 9 ) 2 ; -CO-CMO
- X is -CHR 32 o-, -CHR 32 o-alkyl-, or -CHR 32 o-alkenyl-;
- R120 is selected from: -heteroaryl; -heterocyclyl; -R420- heteroaryl; and -R 42 o-heterocyclyl;
- R220 is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkyl-Y-alkyl; -alkyl- Y- alkenyl; -alkyl- Y-aryl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -CO-C ⁇ -10 alkyl; -CO-O-C MO alkyl; -N 3 ; -aryl; -heteroaryl; -heter
- X is -CHR 52 ⁇ -, -CHR 5 2i -alkyl-, or -CHR 52 ⁇ -alkenyl- Rm is selected from: - m— NR321— SO 2 —R621— alkyl; -R421— R321 — SO 2 — R — alkenyl; -R 42 i-NR 32 i-S ⁇ 2 -R 62 ⁇ -aryl; -R 42 i-NR 32 i-S ⁇ 2 -R 62 ⁇ -heteroaryl; -R 2 ⁇ -NR 32 i-S ⁇ 2 -R 62 i-heterocyclyl; -R421— NR321— SO 2 — R721; -R 42 i-NR 32 i-SO 2 -NR 52 i-R 62 i-alkyl; -R 42 ⁇ -NR 32 i-SO 2 -NR 52 i-R 62 i-al
- R ⁇ 22 is selected from: -Rt22— NR322— SO 2 — R6 22 — alkyl; - r2 2 — R322 — SO2 — R ⁇ 22 — alkenyl; -R 422 -NR 322 -SO 2 -R 622 -aryl; -R 422 -NR 322 -SO 2 -R 6 22-heteroaryl; -R 422 -NR 322 -SO 2 -R 622 -heterocyclyl; -R422 — NR322 — SO 2 — R722; -R 22 -NR 322 -SO 2 -NR 522 -R 622 -alkyl; -R 2 2 -NR 322 -SO 2 -NR 522 -R 622 -alkyl; -R 2 2 -NR 322 -SO 2 -NR 522 -R 622 -alkyl; -R 2 2 -NR
- R 222 is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkyl-Y-alkyl; -alkyl-Y- alkenyl; -alkyl-Y-aryl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -N(R 522 ) 2 ; -CO-N(R 522 ) 2 ; -CO-Ci-io alkyl; -CO-O-CMO alkyl; -N 3 ; -aryl; -heteroaryl; -heterocyclyl; -CO-aryl; and -CO-heteroaryl;
- Y is -O- or -S(O)o- 2 -;
- R 322 is H, CMO alkyl, or arylalkyl; each R- ⁇ 22 is independently alkyl or alkenyl, which may be interrupted by one or more -O- groups; or R 322 and R « 2 can join together to form a ring;
- each R 52 2 is independently H, C O alkyl, or C2-10 alkenyl;
- Rg22 is a bond, alkyl, or alkenyl, which may be interrupted by one or more -O- groups;
- R 722 is CMO alkyl; or R 322 and R 722 can join together to form a ring;
- v is 0 to 4; and each R 22 present is independently selected from C MO alkyl, C MO alkoxy, hydroxy, halogen, and trifluoromethyl;
- X is -CHR 323 -, -CHR 3 3-alkyl-, or -CHR 323 -alkenyl-;
- Z is -S-, -SO-, or-SO 2 -;
- R123 is selected from: -alkyl; -aryl; -heteroaryl; -heterocyclyl; -alkenyl; -R 423 -aryl; -R423- heteroaryl; and -R 423 -heterocyclyl;
- R223 is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkyl-Y-alkyl; - alkyl- Y- alkenyl; -alkyl- Y-aryl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -hal
- X is -CHR 32 -, -CHR 324 -alkyl-, or -CHR 324 -alkenyl-;
- Z is -S-, -SO-, or-SO 2 -;
- Ri2 4 is selected from: -alkyl; -aryl; -heteroaryl; -heterocyclyl; -alkenyl; -R424- heteroaryl; and -R 4 2 4 -heterocyclyl;
- R22 4 is selected from: -hydrogen; -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkyl-Y-alkyl; - alkyl- Y- alkenyl; -alkyl- Y-aryl; and - alkyl or alkenyl substituted by one or more substituents selected from: -OH; -halogen; -N(R
- R 125 is selected from: -R 42 S-NRS 2 S-CR32S— R 525 -Z-R6 25 -alkyl; -R 25 -NR 825 -CR 325 — NR 525 -Z-R 625 -alkenyl; - 125 -NR 525 -CR3 2S — NR 525 -Z-R 625 -aryl; -R42y R825-CR32 ⁇ — NR 525 -Z-R 625 -heteroaryl; -R425-NR825 — CR325 — NR525R725; -R 425 -NR 825 -CR 32 5— R 925 -Z— ⁇ s-alkyl; -R 425 -NR 825 -CR 3 5 — R
- X is -CHR 526 -, -CHR 52 6-alkyl-, or -CHR 526 -alkenyl-;
- R 126 is selected from: -R 126 - RS26-CR326— R 526 -Z-R 626 -alkyl; -R 26 -NR 826 -CR 3 26— -NR 526 -Z-R 626 -alkenyl; -R 2 6 -NR 826 -CR 3 2 6 — NR 526 -Z-R 626 -aryl; -R 4 26- R 826 -CR32 6 — NR 526 -Z-R 626 -heteroaryl; -R 426 -NR 826 -CR 326 — NR 526 -Z-R 626 -heterocyclyl; -R426— NR 8 26— CR 326 — NR 526 R7 2 6; -R 426 -NR 826
- X is alkylene or alkenylene; Y is -CO- or-CS; Z is a bond, -O-, or -S-;
- R 127 is aryl, heteroaryl, heterocyclyl, alkyl or alkenyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from: -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -substituted cycloalkyl; -substituted aryl; -substituted heteroaryl; -substituted heterocyclyl; -O-alkyl; -O-(alkyl) 0 -i-aryl; -O-(alkyl)o- ⁇ -(substituted aryl); -O-(alkyl)o- ⁇ -heteroaryl; -O-(alkyl)o
- R22 7 is selected from: -hydrogen; -alkyl; 20 -alkenyl; -aryl; -substituted aryl; -heteroaryl; -substituted heteroaryl; 25 -alkyl-O-alkyl; -alkyl-S-alkyl; -alkyl-O-aryl; -alkyl-S-aryl: -alkyl-O- alkenyl; 30 -alkyl-S- alkenyl; and -alkyl or
- R 328 and R 28 are independently selected from hydrogen, alkyl, alkenyl, halogen, alkoxy, amino, alkylamino, dialkylamino, and alkylthio; R 528 is H or C MO
- Ri 29 is aryl, heteroaryl, heterocyclyl, alkyl or alkenyl, each of which may be unsubstituted or substituted by one or more substituents independently selected from: -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -substituted cycloalkyl; 5 -substituted aryl; -substituted heteroaryl; -substituted heterocyclyl; -O-alkyl; -O-(alkyl)o- ⁇ -aryl; 10 -O-(alkyl)o- ⁇ -(substituted aryl); -O-(alkyl)o
- R 29 are independently selected from hydrogen, alkyl, alkenyl, halogen, alkoxy, amino, alkylamino, dialkylamino, and alkylthio;
- R 529 is H or C MO alkyl, or R 5 2 9 can join with X to form a ring that contains one or two heteroatoms;
- each R ⁇ 29 is independently H or C ⁇ - ⁇ oalkyl;
- R 729 is H or C MO alkyl which may be interrupted by a heteroatom; or when Ri 29 is alkyl, R ? 2 9 and R12 9 can join to form a ring; and pharmaceutically acceptable salts thereof.
- the IRM compound can be chosen from 1 -position ether or thioether substituted lH-imidazo[4,5-c]pyridm-4-amines defined by Formula XXX below:
- XXX wherein: X is -C ⁇ (R 53 o)-, -CH(R 530 )-alkylene-, -CH(R 530 )-alkenylene- or CH(R 53 o)-alkylene-Y-alkylene-; Y is -O- or-S(O) 0 - 2 -; -W-RBO is selected from -O-RBO-I-5 and -S(O)o- 2 -Ri3o- 6 ; RBO-I-S is selected from -R 63 o-C(R 7 3o)-Z-R 83 o— alkyl; -R 63 o-C(R 7 3o)-Z-R 8 3o— alkenyl; -R 63 o-C(R 7 3o)-Z-R 83 o— aryl; -R 630 -C(R7 3 o)-Z-Rs3o — heteroaryl; -
- R BO - 6 is selected from: -alkyl; -aryl; -heteroaryl; -heterocyclyl; -alkenyl; -R 630 -aryl; -R 6 3o- heteroaryl; and -R 630 -heterocyclyl; each R 53 o is independently hydrogen, CMO alkyl, or C 2 - ⁇ o alkenyl; R ⁇ o is alkylene, alkenylene, or alkynylene, which may be interrupted by one or more -O- groups; R
- R1030 is hydrogen or C M O alkyl; or R 9 3o and R1030 can join together to form a ring selected from RI O is CMO alkyl; or R 9 3o and R 1130 can join together to form a ring having the structure
- R1230 is C 2 - 7 alkylene which is straight chain or branched, wherein the branching does not prevent fonnation of the ring; and R230, R330 and R 4 3o are independently selected from hydrogen and non- interfering substitutents; and pharmaceutically acceptable salts thereof.
- Illustrative non-interfering R230 substituents include: -alkyl; -alkenyl; -aryl; -heteroaryl; -heterocyclyl; -alkylene- Y-alkyl; -alkylene- Y- alkenyl; -alkylene- Y-aryl; and - alkyl or alkenyl substituted by one or more substituents selected from the group consisting of: -OH; -halogen; -N(R 53 o) 2 ; -C(O)-C ⁇ - ⁇ o alkyl; -C(O)-O-CMO alkyl; -N 3 ; -aryl; -heteroaryl; -heterocyclyl; -C(O)-aryl; and -C(O)-heteroaryl.
- Illustrative non-interfering R 33 o and R4 3 o substitutents include: CMO alkyl, C2-10 alkenyl, C2-10 alkynyl, CMO alkoxy, C MO alkylthio, amino, alkylamino, dialkylamino, halogen, and nitro.
- the IRM compound can be chosen from IH-imidazo dimers of the formula (XXXI):
- A is a divalent linking group selected from the group consisting of: sfraight or branched chain C 4 _ 2 o alkylene; straight or branched chain C 4 .2o alkenylene; straight or branched chain C 4 _2o alkynylene; and -Z-Y-W-Y-Z-; each Z is independently selected from the group consisting of: straight or branched chain C2-20 alkylene; straight or branched chain C -2o alkenylene; and straight or branched chain C 4 .
- each Y is independently selected from the group consisting of: a bond; -N(R 53 ⁇ )C(O)-; -N(R 53 ⁇ )C(O)N(R 53 ⁇ )-; - N(R 53 ⁇ )S(O) 2 -; -S(O) 2 N(R 53 ⁇ )-; -OC(O)O-; -OC(O)-; -C(O)O-; -N(R 53 ⁇ )C(O)O-; and -OC(O)N(R 531 )-;
- W is selected from the group consisting of: straight or branched chain C 2 - 20 alkylene; straight or branched chain C 2 - 2 o alkenylene; straight or branched chain C 4 . 20 alkynylene; straight or branched chain perfluoro C 2 - 2 o alkylene; C alkylene; -C(O)-; -S(O) 2 -; -OC(O)O-; -N(R 53 i)C(O)N(R 53 i)-;
- trans-5-norbornen-2,3-diyl wherein n is 0 - 4; each R is independently selected from the group consisting of C ⁇ - 4 alkyl, C ⁇ -4 alkoxy, and halogen; and Q is selected from the group consisting of a bond, -CH 2 -, and -O-;
- R 2 ⁇ is selected from the group consisting of: -hydrogen; -alkyl; -alkenyl; -aryl; -substituted aryl; -heteroaryl; -substituted heteroaryl; -alkyl-X-alkyl; -alkyl-X-aryl; -alkyl-X- alkenyl; and -alkyl or alkenyl substituted by one or more substituents selected from the group consisting of: -OH; -halogen; -N(R 63 ⁇ ) 2 ; -C(O)-N(R 63 ⁇ ) 2 ; -C(S)-N(R 63 ⁇ ) 2 ; -N(R 63 i)-C(O)-Ci.,o alkyl; -N(R63I)-C(S)-CMO alkyl; -N(R63i)- S(O) 2 -CMO alkyl
- each R ⁇ ⁇ is independently hydrogen or C MO alkyl; R 73 ⁇ is C . 8 alkylene; and X is -O- or -S-; with the proviso that if W is -C(O)-, -S(O) 2 -, -OC(O)O-, or -N(R 53! )C(O)N(R 531 )- then each Y is a bond; and pharmaceutically acceptable salts thereof.
- the IRM compound can be chosen from 6-, 1-, 8-, or 9- position aryl or heteroaryl substituted lH-imidazo[4,5-c]quinolin-4-amines of the following Formula (XXXII):
- R 32 is selected from the group consisting of alkyl, alkoxy, hydroxy, and trifluoromethyl; n is 0 or 1 ; R B2 and R232 are independently selected from the group consisting of hydrogen and non-interfering substitutents; R 33 2 is selected from the group consisting of: -Z-Ar, -Z-Ar'-X-Y-R 432 , -Z-Ar'-X-R 532 ; Ar is selected from the group consisting of aryl and heteroaryl both of which can be unsubstituted or can be substituted by one or more substituents independently selected from the group consisting of alkyl, alkenyl, alkoxy, methylenedioxy, haloalkyl, haloalkoxy, halogen, nitro, hydroxy, hydroxyalkyl, mercapto, cyano, carboxy, formyl, aryl, aryloxy, arylalkoxy, heteroaryl, heteroaryloxy,
- Ri 32 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl,
- A is selected from the group consisting of -O-, -C(O)-, -S(O)o- 2 -, -CH 2 -, and - N(R4 32 )-;
- W is selected from the group consisting of a bond, -C(O)-, and -S(O)
- each X is independently selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated with arylene, heteroarylene, or heterocyclylene, and optionally interrupted by one or more -O- groups; each Y is independently selected from the group consisting of: -S(O)o-2-, -C(R 632 )-, -C(R 632 )-O-, -O-C(O)-O-, -N(R 832 )-Q-,
- R 43 2 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, hydroxyalkyl, haloalkyl, haloalkoxy, halogen
- each W is mdependently selected from the group consisting of a bond, -C(O)-, and-S(O) 2 -; and a and b are independently integers from 1 to 6 with the proviso that a + b is ⁇ 7;
- Illustrative non-interfering R232 substitutents include:
- X is selected from the group consisting of alkylene, alkenylene, alkynylene, arylene, heteroarylene, and heterocyclylene wherein the alkylene, alkenylene, and alkynylene groups can be optionally interrupted or terminated with arylene, heteroarylene, or heterocyclylene, and optionally interrupted by one or more -O- groups;
- Y is selected from the group consisting of: -S(O) 2 -N(R 832 )-,
- R 432 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents mdependently selected from the group consisting of alkyl, alkoxy, hydroxyalkyl, haloalkyl, haloalkoxy,
- A is selected from the group consisting of -O-, -C(O)-, -S(O)o- 2 -, -CH 2 -, and - N(R4 32 )-;
- W is selected from the group consisting of a bond, -C(O)-, and -S(O
- alkyl As used herein, the terms "alkyl”, “alkenyl”, “alkynyl” and the prefix “alk-” are inclusive of both straight chain and branched chain groups and of cyclic groups, i.e. cycloalkyl and cycloalkenyl. Unless otherwise specified, these groups contain from 1 to
- alkenyl and alkynyl groups containing from 2 to 20 carbon atoms. In some embodiments, these groups have a total of up to 10 carbon atoms, up to 8 carbon atoms, up to 6 carbon atoms, or up to 4 carbon atoms.
- Cyclic groups can be monocyclic or polycyclic and preferably have from 3 to 10 ring carbon atoms. Exemplary cyclic groups include cyclopropyl, cyclopropylmethyl, cyclopentyl, cyclohexyl, adamantyl, and substituted and unsubstituted bornyl, norbornyl, and norbornenyl.
- alkylene alkenylene
- alkynylene alkynylene
- an arylalkenyl group comprises an alkylene moiety to which an aryl group is attached.
- haloalkyl is inclusive of groups that are substituted by one or more halogen atoms, including perfluorinated groups. This is also true of other groups that include the prefix "halo-”. Examples of suitable haloalkyl groups are chloromethyl, trifluoromethyl, and the like.
- aryl as used herein includes carbocyclic aromatic rings or ring systems.
- aryl groups include phenyl, naphthyl, biphenyl, fluorenyl, and indenyl.
- hetero atom refers to the atoms O, S, or N.
- heteroaryl includes aromatic rings or ring systems that contain at least one ring hetero atom.
- Suitable heteroaryl groups include furyl, thienyl, pyridyl, quinolinyl, isoquinolinyl, indolyl, isoindolyl, triazolyl, pyrrolyl, tetrazolyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, benzofuranyl, benzothiophenyl, carbazolyl, benzoxazolyl, pyrimidinyl, benzimidazolyl, quinoxalinyl, benzothiazolyl, naphthyridinyl, isoxazolyl, isothiazolyl, purinyl, quinazolinyl, pyrazinyl, 1- oxidopyridyl, pyridazinyl, triazinyl, tetrazinyl, oxadiazolyl, thiadiazolyl, and so on.
- heterocyclyl includes non-aromatic rings or ring systems that contain at least one ring hetero atom and includes all of the fully saturated and partially unsaturated derivatives of the above mentioned heteroaryl groups.
- exemplary heterocyclic groups include pyrrolidinyl, tefrahydrofuranyl, mo ⁇ holinyl, thiomo ⁇ holinyl, piperidinyl, piperazinyl, thiazolidinyl, imidazolidinyl, isothiazolidinyl, tefrahydropyranyl, quinuclidinyl, homopiperidinyl, homopiperazinyl, and the like.
- arylene “heteroarylene,” and “heterocyclylene” are the divalent forms of the "aryl,” “heteroaryl,” and “heterocyclyl” groups defined above.
- arylenyl “heteroarylenyl,” and “heterocyclylenyl” are the divalent forms of the "aryl,” “heteroaryl,” and “heterocyclyl” groups defined above.
- an alkylarylenyl group comprises an arylene moiety to which an alkyl group is attached.
- the aryl, heteroaryl, and heterocyclyl groups of Formulas IX - XXX can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, methylenedioxy, ethylenedioxy, alkylthio, haloalkyl, haloalkoxy, haloalkylthio, halogen, nitro, hydroxy, mercapto, cyano, carboxy, formyl, aryl, aryloxy, arylthio, arylalkoxy, arylalkylthio, heteroaryl, heteroaryloxy, heteroarylthio, heteroarylalkoxy, heteroarylalkylthio, amino, alkylamino, dialkylamino, heterocyclyl, heterocycloalkyl, alkylcarbonyl, alkenylcarbonyl, alkoxycarbonyl, haloalkylcarbonyl, halo
- the IRM compounds and salts thereof described herein include any of their pharmaceutically acceptable forms, such as isomers (e.g., diastereomers and enantiomers), solvates, polymo ⁇ hs, and the like.
- the invention specifically includes the use of each of the compound's enantiomers as well as racemic mixtures of the enantiomers.
- the topical formulations of the present invention are prepared using the free base form of the IRM compound.
- the IRM is an imidazonaphthyridine amine.
- the IRM is 2-methyl-l-(2-methylpropyl)-lH-imidazo[4,5- c] [1 ,5]naphthyridin-4-amine.
- the amount of an IRM compound that will be therapeutically effective in a specific situation will depend on such things as the activity of the particular compound, the dosing regimen, the application site, the particular formulation and the condition being treated.
- a therapeutically effective amount means an amount of the compound sufficient to induce a therapeutic or prophylactic effect, such as cytokine induction, inhibition of TH2 immune response, antiviral or antitumor activity, reduction or elimination of postsurgical scarring, reduction or resolution of actinic keratosis or pre-actinic keratosis lesions, reduction in the recurrence of actinic keratosis, or protection against uv-induced epidermal neoplasia, or as an adjuvant for therapeutic and prophylactic vaccines, including DNA, whole cell, protein subunit, attenuated virus, and all other vaccines, where the formulation may be applied before, during and/or after vaccine delivery.
- a therapeutic or prophylactic effect such as cytokine induction, inhibition of TH2 immune response, antiviral or antitumor activity, reduction or elimination of postsurgical scarring, reduction or resolution of actinic keratosis or pre-actinic keratosis lesions, reduction
- the amount of the IRM compound present in a topical formulation of the invention will be an amount effective to treat a targeted condition, to prevent recurrence of the condition, or to promote immunity against the condition.
- the amount or concentration of the IRM compound is at least 0.0001% by weight, such as, for example, at least 0.001%, at least 0.003%, at least 0.005%, at least 0.01%, at least 0.03%, at least 0.10%, at least 0.20%, at least 0.25%, at least 0.27%, at least 0.30%, and at least 1.0%, by weight based on the total weight of the formulation.
- the amount of the IRM compound is at most 10% by weight, such as, for example, at most 5.0%, at most 3.0%, at most 1.0%, at most 0.5%, at most 0.4%, at most 0.35%, at most 0.33%, and at most 0.3%, by weight based on the total weight of the formulation.
- the formulation includes a preservative system.
- the preservative system includes one or more compounds that inhibit microbial growth (e.g., fungal and bacterial growth) within the formulation (for example, during manufacturing and use).
- the preservative system includes at least one preservative compound chosen from sorbic acid, esters or salts thereof, such as, for example, isopropyl sorbate, calcium sorbate, potassium sorbate, sodium sorbate, and triethanolammonium sorbate. Combinations of these may be used in formulations of the present invention. Such preservatives adversely affect the stability of the formulations as described herein.
- the sorbic acid preservative i.e., sorbic acid, esters or salts thereof, or combinations thereof
- a formulation in an amount of at least 0.005% by weight, more preferably at least 0.01% by weight, even more preferably at least 0.02% by weight, even more preferably at least 0.05% by weight, and even more preferably at least 0.08% by weight, based on the total weight of the formulation.
- the sorbic acid preservative is preferably present in a formulation in an amount of no greater than 1% by weight, more preferably no greater than 0.5% by weight, even more preferably no greater than 0.2% by weight, even more preferably no greater than 0.12% by weight, and even more preferably, no greater than 0.10% by weight, based on the total weight of the formulation.
- the preservative system will generally include at least one additional (i.e., secondary) preservative compound, such as, for example, methylparaben, ethylparaben, propylparaben, butylparaben, and phenoxyethanol. Various combinations of these compounds can be included in the preservative system.
- the secondary preservative compound is methylparaben. In some embodiments of the invention, the secondary preservative compound is present in an amount of at least 0.01% by weight, such as for example, at least 0.02%, at least 0.03%, at least 0.04%, and at least 0.05%, by weight based on the total weight of the formulation. In other embodiments of the invention the secondary preservative compound is present in an amount of at most 0.5 %, such as for example, at most 0.4%, at most 0.3%, and at most 0.2%, by weight based on the total weight of the formulation.
- the preservative system may also include a preservative enhancing solubilizer which enhances the solubility of the preservative in the aqueous phase, examples of which include diethylene glycol monoethyl ether, propylene glycol, and polyethylene glycol)(4) monolaurate. Combinations of such enhancing solubilizers can be used in formulations of the present invention.
- propylene glycol is present in an amount of at least 1.0% by weight, such as for example, at least 2.0%, at least 3.0%, at least 4.0%, and at least 5.0%, by weight based on the total weight of the formulation.
- propylene glycol is present in at most 10.0% by weight, such as for example, at most 8.0%, at most 6.0%, and at most 5.0%, by weight based on the total weight of the formulation.
- Antioxidants suitable for use herein are those that inhibit the autoxidation of the sorbic acid preservative.
- antioxidants having hydrogen atom o donating functionality have demonstrated much greater improvement than others.
- autoxidation intermediates typically, radicals
- Suitable antioxidants are those that are pharmaceutically acceptable and5 described in the International Cosmetic Ingredient Dictionary and Handbook, Ninth Edition, Volume 4, 2002, and in the USP NF 2004: The United States Pharmacopeia, 27 th Revision and The National Formulary, 22 nd Edition.
- antioxidants examples include ascorbic acid (D and/or L enantiomers), ascorbyl palmitate (D and or L enantiomers), butylated hydroxyanisole0 (BHA), butylated hydroxytoluene (BHT), cysteine (D and/or L enantiomers), propyl gallate, sodium formaldehyde sulfoxylate, sodium thiosulfate, sulfur dioxide, tocopherol, including all of its stereoisomers, and tocopherol polyethylene glycol 1000 succinate, including all of its stereoisomers.
- Prefened antioxidants are those containing hydrogen atom donating functional5 groups.
- antioxidants examples include ascorbic acid, ascorbyl palmitate, BHT, BHA, cysteine, propyl gallate, sodium formaldehyde sulfoxylate, tocopherol including all of its stereoisomers, and tocopherol polyethylene glycol 1000 succinate, including all of its stereoisomers.
- More prefened antioxidants are those containing aromatic hydroxy groups0 capable of hydrogen atom donation. Examples of such antioxidants include BHA, BHT, propyl gallate, tocopherol, including all of its stereoisomers, and tocopherol polyethylene glycol 1000 succinate, including all of its stereoisomers. Most prefened antioxidants are BHA and BHT, which can be used in combination.
- the antioxidant is preferably present in a formulation in an amount of at least 0.001% by weight, more preferably at least 0.005% by weight, even more preferably at least 0.008% by weight, and even more preferably at least 0.01% by weight, based on the total weight of the formulation.
- the antioxidant is preferably present in a formulation in an amount of no greater than 0.3% by weight, more preferably no greater than 0.2% by weight, and even more preferably no greater than 0.012% by weight, and even more preferably no greater than 0.1% by weight, based on the total weight of the formulation.
- the sorbic acid preservative (i.e., sorbic acid ester/salt) to antioxidant weight ratio is preferably at least 1 :20, more preferably at least 1:1, and even more preferably at least 5:1.
- the sorbic acid to antioxidant weight ratio is preferably no greater than 1000: 1 , more preferably no greater than 20: 1 , and even more preferably no greater than 10: 1.
- the formulation can also include at least one chelating agent.
- the chelating agent functions to stabilize the antioxidant(s) present in the formulation.
- Chelating agents are compounds that complex with metal ions. Suitable chelating agents are those that are pharmaceutically acceptable and described in the International Cosmetic Ingredient Dictionary and Handbook, Ninth Edition, Volume 4,
- Suitable chelating agents include ethylenediaminetetraacetic acid (EDTA) and citric acid, hydrates thereof, salts thereof, and hydrates of the salts thereof.
- EDTA ethylenediaminetetraacetic acid
- Examples of such chelating agents include ethylenediaminetetraacetic acid disodium salt, ethylenediaminetetraacetic acid disodium salt dihydrate, and citric acid monohydrate.
- EDTA ethylenediaminetetraacetic acid
- citric acid monohydrate ethylenediaminetetraacetic acid disodium salt
- Various combinations of chelating agents can be used if desired.
- the chelating agent is preferably present in a formulation in an amount of at least 0.001% by weight, more preferably at least 0.005% by weight, even more preferably at least 0.01% by weight, and even more preferably at least 0.05% by weight, based on the total weight of the formulation.
- the chelating agent is preferably present in a formulation in an amount of no greater than 0.2% by weight, and more preferably no greater than 0.1% by weight, based on the total weight of the formulation.
- the antioxidant to chelating agent weight ratio is preferably at least 1 :200, more preferably at least 1:10, and even more preferably at least 1:5.
- the antioxidant to chelating agent weight ratio is preferably no greater than 300:1, more preferably no greater than 10:1, and even more preferably no greater than 2:1.
- the topical formulations of the invention can additionally include a fatty acid.
- fatty acid means a carboxylic acid, either saturated or unsaturated having 6 to 28 carbon atoms, such as, for example, from 10 to 22 carbon atoms.
- Non-limiting examples of such fatty acids include isostearic acid, oleic acid, and linear- or branched-chain carboxylic acids of 6 to 18 carbon atoms.
- the fatty acid may be present in the formulation in an amount sufficient to solubilize the IRM compound.
- the amount of the fatty acid is at least 0.05% by weight, at least 1.0% by weight, at least 3.0% by weight, at least 5.0% by weight, at least 6.0% by weight, at least 7.0% by weight, at least 10% by weight, at least 15% by weight, or at least 25% by weight, based on the total weight of the formulation. In certain embodiments, the amount of the fatty acid is at most 40% by weight, at most 30% by weight, at most 15% by weight, at most 10% by weight, or at most 8.0% by weight based on the total weight of the formulation.
- the fatty acid component of the formulation can comprise one or more fatty acids.
- the topical formulations of the invention can additionally include at least one hydrophobic, aprotic component miscible with the fatty acid and comprising a hydrocarbyl group of 7 or more carbon atoms.
- hydrophobic is meant that the component is essentially insoluble in water, i.e. immiscible with water and unable to form a micelle in water, and does not contain polyoxyethylene or acid salt groups.
- the hydrophobic, aprotic component has a hydrophilic lipophilic balance (HLB) of less than 2.
- HLB of a component may be determined as described, for example, in Attwood, D., Florence, A. T. Surfactant Systems: Their Chemistry,
- aprotic is meant that the component cannot donate a proton to the IRM and does not contain groups such as carboxyl, hydroxy, primary and secondary amino, primary and secondary amido, or quaternary ammonium groups.
- this component has a pKa of at least 14.2 and does not substantially solubilize or form a complex such as an acid-base pair or complex or a hydrogen bond complex with the IRM compound.
- not substantially is meant that the ratio of the IRM compound's solubility in the hydrophilic, aprotic component to that in isostearic acid is less than 1:40.
- Formulations intended for dermal or topical use typically have amounts of an oil phase and a hydrophobic, aprotic component sufficient to provide desirable qualities such as spreadability and feel.
- useful hydrophobic, aprotic components include but are not limited to fatty acid esters, for example, isopropyl mysristate, isopropyl palmitate, diisopropyl dimer dilinoleate; medium-chain (e.g., 8 to 14 carbon atoms) triglycerides, for example, caprylic/capric triglyceride; cetyl esters; hydrocarbons of 8 or more carbon atoms, for example, light mineral oil, white petrolatum; and waxes, for example, beeswax.
- fatty acid esters for example, isopropyl mysristate, isopropyl palmitate, diisopropyl dimer dilinoleate
- the hydrophobic, aprotic component is chosen from one or more of isopropyl mysristate, isopropyl palmitate, caprylic/capric triglyceride, and diisopropyl dimer dilinoleate. Various combinations of such hydrophobic, aprotic components can be used if desired.
- the amount of the hydrophobic, aprotic component is at least 1.0% by weight, at least 3.0% by weight, at least 3.5% by weight, at least 4.0% by weight, at least 4.5% by weight, at least 5.0% by weight, or at least 10% by weight, based on the total weight of the formulation.
- the amount of the hydrophobic, aprotic component is at most 30% by weight, at most 15% by weight, at most 10% by weight, or at most 5.0% by weight based on the total weight of the formulation.
- the weight ratio of the hydrophobic, aprotic component to the fatty acid can be 0.025:1 to 600:1, for example, 0.5:1 to 50:1, and 2:1 to 30:1.
- the combined amount (weight percent of the total topical formulation weight) of the hydrophobic, aprotic component and the fatty acid can be 2% to 50% by weight, for example 2% to 30%, 5% to 30%, 5% to 20%, and 10% to 20%.
- the fonnulations of the present invention can also comprise a viscosity enhancing agent.
- the viscosity enhancing agent When water is the continuous phase, the viscosity enhancing agent will be a hydrophilic viscosity enhancing agent.
- suitable hydrophilic viscosity enhancing agents include cellulose ethers such as hydroxypropylmethylcellulose, hydroxyetliylcellulose, hydroxypropylcellulose, and carboxymethylcellulose; polysaccharide gums such as xanthan gum; and homopolymers and copolymers of acrylic acid crosslinked with allyl sucrose or allyl pentaerythriol such as those polymers designated as carbomers in the United States Pharmacopoeia.
- Suitable carbomers include, for example, those available as CARBOPOL 934P,
- CARBOPOL 97 IP, CARBOPOL 940, CARBOPOL 974P, CARBOPOL 980, and PEMULEN TR-1 (USP/NF Monograph; Carbomer 1342), all available from Noveon,
- the viscosity enhancing agent is chosen from CARBOPOL 974P and 980.
- the amount of the viscosity enhancing agent, when used is at least 0.1% by weight, at least 0.2% by weight, at least 0.5% by weight, at least 0.6% by weight, at least 0.7% by weight, at least 0.9% by weight, or at least 1.0% by weight, based on the total weight of the formulation.
- the amount of the viscosity enhancing agent, when used is at most 10% by weight, at most 5.0% by weight, at most 3.0% by weight, at most 2.0% by weight, or at most 1.5% by weight, based on the total weight of the formulation.
- Emulsifiers of the invention can additionally comprise an emulsifier.
- Suitable emulsifiers include non-ionic surfactants such as, for example, polysorbate 60, sorbitan monostearate, polyglyceryl-4 oleate, polyoxyethylene(4) lauryl ether, etc.
- the emulsifier is chosen from poloxamers (e.g., PLURONIC F68, also known as POLOXAMER 188, a poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol), available from BASF, Ludwigshafen, Germany) and sorbitan trioleate (e.g., SPAN 85 available from Uniqema, New Castle, DE).
- the emulsifier is generally present in an amount of 0.1% to 10% by weight of total formulation weight, for example, from 0.5% to 5.0% by weight, and from 0.75% to 3.5% by weight. In certain embodiments, the amount of the emulsifier, if used, is present in an amount of at least 0.1% by weight, at least 0.5% by weight, at least 0.75% by weight, at least 1.0% by weight, at least 2.5% by weight, at least 3.5% by weight, or at least 5.0% by weight, based on the total weight of the formulation. In certain embodiments, the amount of the emulsifier, if used, is present in an amount of at most 10% by weight, at most 5.0% by weight, or at most 3.5% by weight, based on the total weight of the formulation.
- the formulations of the present invention may additionally include at least one pH adjuster.
- Suitable pH adjusters include organic bases and inorganic bases such as, for example, KOH and NaOH (e.g., aqueous formulations).
- the pH of the topical formulations of the present invention generally ranges from 3.5 to 7.0. In one embodiment, the pH of the topical formulations of the present invention can range from 4.0 to 6.0, preferably 5.0.
- Illustrative Formulations Prefened fonnulations of the present invention are as follows. The water used is typically purified water.
- a pharmaceutical formulation includes: 0.001% by weight to 5.0% by weight of an imidazonaphthyridine amine (preferably, 2-methyl-l-(2-metl ylpropyl)-lH-imidazo[4,5-c][l,5]naphthyridin-4- amine); 0.02% by weight to 0.2% by weight of a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof; 0 to 10.0% by weight of propylene glycol; 0.05% by weight to 0.2% by weight of methylparaben; 0.001% by weight to 0.2% by weight of butylated hydroxyanisole, butylated hydroxytoluene, or combinations thereof; 0 to 0.1% by weight of ethylenediaminetetraacetic acid, a hydrate thereof, a salt thereof, a hydrate of a the salt thereof, or combinations thereof; 1 % by weight to 30% by weight of an imidazonap
- a pharmaceutical formulation includes: 0.3% by weight of 2-methyl-l-(2-methylpropyl)-lH-imidazo[4,5- c][l,5]naphthyridin-4-amine; 0.15% by weight sorbic acid; 5.0% by weight propylene glycol; 0.2% by weight methylparaben; 0.1% by weight butylated hydroxyanisole; 0.05% by weight ethylenediaminetetraacetic acid disodium salt dihydrate;
- a pharmaceutical formulation includes: 0.30% by weight of 2-methyl-l-(2-methylpropyl)-lH-imidazo[4,5-aphthyridin-4-amine;
- caprylic/capric triglyceride 4.00% by weight of caprylic/capric triglyceride
- a pharmaceutical formulation includes: 0.3% by weight of 2-methyl-l-(2-methyl ⁇ ropyl)-lH-irnidazo[4,5- aphthyridin-4-amine;
- caprylic/capric triglyceride 4.0% by weight of caprylic/capric triglyceride; 1.0% by weight of a carbomer;
- a pharmaceutical formulation includes:
- sorbic acid 0.15% by weight sorbic acid; 5.0% by weight propylene glycol;
- a pharmaceutical formulation includes: 0.1% by weight of 2-methyl-l-(2-methylpropyl)-lH-imidazo[4,5- c] [ 1 ,5]naphthyridin-4-amine; 0.15% by weight sorbic acid; 5.0% by weight propylene glycol; 0.2% by weight methylparaben; 0.1% by weight butylated hydroxyanisole; 0.05% by weight ethylenediaminetetraacetic acid disodium salt dihydrate; 5.0% by weight isostearic acid; 4.0% by weight of caprylic/capric triglyceride; 1.0% by weight of a carbomer; 3.5% by weight of a poloxamer; 0.8% by weight of an aqueous solution of 20% by weight NaO ⁇ in water; and 80.1 % by weight water; wherein the weight percentages are based on the total weight of the formulation.
- Formulations according to the present invention can be applied to any suitable location, for example topically to dermal and/or mucosal surfaces, or internally to a particular tissue location.
- the therapeutic effect of the IRM compound may extend only to the superficial layers of the dermal surface or to tissues below the dermal surface.
- another aspect of the present invention is directed to a method for the treatment of a dermal and/or mucosal associated condition comprising applying to skin one of the foregoing formulations.
- a "dermal and/or mucosal associated condition” means an inflammatory, infectious, neoplastic or other condition that involves a dermal and/or mucosal surface or that is in sufficient proximity to a dermal and/or mucosal surface to be affected by a therapeutic agent topically applied to the surface.
- a dermal and/or mucosal associated condition include warts, atopic dermatitis, postsurgical scars, lesions caused by a he ⁇ es virus, and epidermal neoplasias, such as for example actinic keratosis, pre- actinic keratosis lesions, malignant melanomas, basal cell carcinoma, and squamous cell carcinoma.
- the formulations can be applied to the surface of skin for treatment of actinic keratosis (AK).
- Actinic keratoses are premalignant lesions considered biologically to be either carcinoma in-situ or squamous intraepidermal neoplasia.
- AK is the most frequent epidermal tumor and is induced by ultraviolet (UV) radiation, typically from sunlight. Because of its precancerous nature, AK may be o considered the most important manifestation of sun-induced skin damage.
- UV ultraviolet
- the above described formulations are particularly advantageous for dermal and/or mucosal application for a period of time sufficient to obtain a desired therapeutic effect without undesired systemic abso ⁇ tion of the IRM.
- the precise amount of formulation effective for treating a dermal and/or5 mucosal associated condition will vary according to factors known in the art including but not limited to the particular IRM compound, the particular formulation, the intended dosing regimen, the particular condition being treated, the state of the subject's immune system (e.g., suppressed, compromised, stimulated), and the species to which the formulation is being administered.
- the amount of formulation is0 an amount sufficient to deliver a dose of about 0.02 mg to about 15 mg of IRM compound.
- the amount of formulation is an amount sufficient to deliver a dose of about 0.2 mg to about 2.5 mg of IRM compound.
- the amount of formulation is an amount sufficient to deliver a dose of about 0.5 mg to about 1.7 mg of IRM compound.
- a dose5 of 0.75 mg of IRM compound is delivered. In another particular embodiment a dose of 1.5 mg of IRM compound is delivered.
- the dosing regimen will vary at least in part on many factors known in the art including but not limited to the particular IRM compound, the particular formulation, the amount of formulation being administered, the particular condition being treated,0 the state of the subject's immune system (e.g., suppressed, compromised, stimulated), and the species to which the formulation is being administered.
- the formulation is administered at least once a week, at least twice a week, or at least three times a week. In other embodiments the formulation is administered at most seven times a week, at most six times a week, at most five times a week, or at most four times a week.
- the formulation is administered for at least two weeks, for at least four weeks, for at least six weeks, or for at least eight weeks. In other embodiments the formulation is administered for at most sixteen weeks, for at most twelve weeks, or for at most eight weeks. In some embodiments, about 200 to about 600 mg of formulation is administered twice a week for eight weeks. In one particular embodiment, about 250 mg of the formulation described in Example 22 below is administered twice a week for eight weeks. In another particular embodiment, about 500 mg of the formulation described in Example 22 below is administered twice a week for eight weeks.
- ⁇ PLC parameters Analytical column: ZORBAX RX-C8, 5.0 micron particle, 150 x 4.6 mm (available from Agilent Technologies, Wilmington, Delaware, USA); Column temperature: 30°C; Detector: UV at 254 nm; Flow Rate: 1.0 mL/min; Injection volume: 25 ⁇ L; Mobile phase A: 62% aqueous (0.2% sodium 1-octanesulfonate, 0.2% triethylamine, 0.2% of 85% phosphoric acid), 21% acetonitrile, 17% methanol; Mobile Phase B: 20% aqueous (0.2% sodium 1-octanesulfonate, 0.2% triethylamine, 0.2% of 85% phosphoric acid), 42% acetonitrile, 38% methanol; Data acquisition time: 23 minutes; HPLC run time: approximately 30 minutes.
- IRM Compound 1 sample solution A portion of the cream formulation (1000 mg for creams containing 0.01, 0.03, 0.05 and 0.1% IRM and 250 mg for creams containing 0.3, 0.6, and 1.0% IRM) was accurately weighed into a volumetric flask (50 mL for the 1000 mg samples and 100 mL for the 250 mg samples). Approximately 40 mL of diluent (prepared by combing 200 parts of acetonitrile, 790 parts water, and 10 parts phosphoric acid, all parts by volume) was added to the 50 mL flask or 80 mL to the 100 mL flask. The flask was sonicated with occasional shaking for 20 minutes or until the cream was completely dispersed.
- diluent prepared by combing 200 parts of acetonitrile, 790 parts water, and 10 parts phosphoric acid, all parts by volume
- Sorbic acid sample solution A 250 mg portion of cream was accurately weighed into a 100 mL volumetric flask. Approximately 80 mL of diluent (prepared by combing 200 parts of acetonitrile, 790 parts water, and 10 parts phosphoric acid, all parts by volume) was added to the flask. The flask was sonicated with occasional shaking for 20 minutes or until the cream was completely dispersed. The solution was allowed to cool to ambient temperature and then diluted to volume with diluent.
- Test Method 2 A gradient reversed phase high performance liquid chromatography (HPLC) method was used to determine the amount of BHA in cream formulations containing IRM Compound 1.
- HPLC parameters Analytical column: ZORBAX Bonus RP, 3.5 micron particle, 150 x 3.0 mm; Column temperature: 40°C; Detector: UV at 290 nm; Flow Rate: 0.5 mL/min; Injection volume: 20 ⁇ L; Mobile phase A: 0.1% formic acid in water; Mobile Phase B: 0.05% formic acid in acetonitrile; Data acquisition time: 12 minutes; HPLC run time: approximately 20 minutes. Gradient program: 0 minutes: 60% mobile phase A, 40% mobile phase B; 10 minutes: 5% mobile phase A, 95% mobile phase B; 12 minutes: 5% mobile phase A, 95% mobile phase B; 13 minutes: 60% mobile phase A, 40% mobile phase B; 20 minutes: 60% mobile phase A, 40% mobile phase B.
- Sample solution A portion (approximately 1000 mg) of the cream formulation was accurately weighed into a 100 mL volumetric flask. Approximately 80 mL of diluent (prepared by combining 600 parts of acetonitrile, 400 parts of water, and 1 part formic acid, all parts by volume) was added and the flask was sonicated with occasional shaking for 10 minutes or until the cream was well dispersed. The solution was allowed to cool to ambient temperature and then diluted to volume with diluent. A portion of the solution was filtered using a syringe equipped with a 0.2 micron PTFE filter to provide the sample solution.
- diluent prepared by combining 600 parts of acetonitrile, 400 parts of water, and 1 part formic acid, all parts by volume
- Test Method 3 IRM Compound 1 Content
- HPLC high performance liquid chromatography
- ⁇ PLC parameters Analytical column: ZORBAX Bonus RP, 3.5 micron particle, 150 x 4.6 mm (available from Agilent Technologies, Wilmington, Delaware, USA); Column temperature: 35°C; Detector: UV at 240 nm; Flow Rate: 1.0 mL/min; Injection volume: 30 ⁇ L; Mobile phase A: 0.05% frifluoroacetic acid in water; Mobile Phase B: 0.05% frifluoroacetic acid in acetonitrile; Data acquisition time: 25 minutes; HPLC run time: 35 minutes.
- Gradient program 0 minutes: 80% mobile phase A, 20% mobile phase B; 5 minutes: 80% mobile phase A, 20% mobile phase B; 15 minutes: 75% mobile phase A, 25% mobile phase B; 25 minutes: 35% mobile phase A, 65% mobile phase B; 28 minutes: 10% mobile phase A, 90% mobile phase B; 29 minutes: 80% mobile phase A, 20% mobile phase B; 35 minutes: 80% mobile phase A, 20% mobile phase B.
- Sample solution A portion of the cream formulation (2500 mg for creams containing 0.03% IRM; 1500 mg for creams containing 0.1% IRM; and 500 mg for creams containing 0.3% IRM) was accurately weighed into a volumetric flask (50 mL for creams containing 0.03% IRM; 100 mL for creams containing 0.1 or 0.3% IRM). Approximately 40 mL of diluent (prepared by combing 200 parts of acetonitrile, 790 parts water, and 10 parts phosphoric acid, all parts by volume) was added to the 50 mL flask or 80 mL to the 100 mL flask.
- diluent prepared by combing 200 parts of acetonitrile, 790 parts water, and 10 parts phosphoric acid, all parts by volume
- the flask was shaken or vortexed to dislodge any cream from the neck of the flask and then sonicated with occasional shaking for 10 minutes or until the cream was completely dispersed.
- the solution was allowed to cool to ambient temperature and then diluted to volume with diluent. A portion of the solution was filtered using a syringe equipped with a 0.2 micron PTFE filter to provide the sample solution.
- HPLC high performance liquid chromatography
- Sample solution A portion (approximately 1000 mg) of the cream formulation was accurately weighed into a 100 mL volumetric flask. Approximately 80 mL of diluent (prepared by combining 600 parts of acetonitrile, 400 parts of water, and 1 part frifluoroacetic acid, all parts by volume) was added and the flask was sonicated with occasional shaking for 10 minutes or until the cream was well dispersed. The solution was allowed to cool to ambient temperature and then diluted to volume with diluent. A portion of the solution was filtered using a syringe equipped with a 0.45 micron PTFE filter to provide the sample solution.
- Cream Formulations The cream formulations in the Examples below were prepared using the following general method.
- Oil phase preparation The IRM compound and the BHA or BHT were dissolved in the isostearic acid and medium chain triglycerides, with heat if necessary.
- CARBOPOL 980 was then dispersed in the oil phase.
- Water phase preparation Edetate disodium dihydrate, methylparaben, sorbic acid, propylene glycol, and POLOXAMER 188 were added to the water and mixed until dissolved, with heat if necessary. If the CARBOPOL was not dispersed in the oil phase, it was dispersed in the water phase.
- Phase combination The oil phase was added to the water phase at ambient conditions. The emulsion was then homogenized.
- Table 1 summarizes topical formulations made in accordance with the present invention in a percentage weight-by-weight basis. The formulations were packaged in aluminum tubes with an epoxy phenolic lacquer liner.
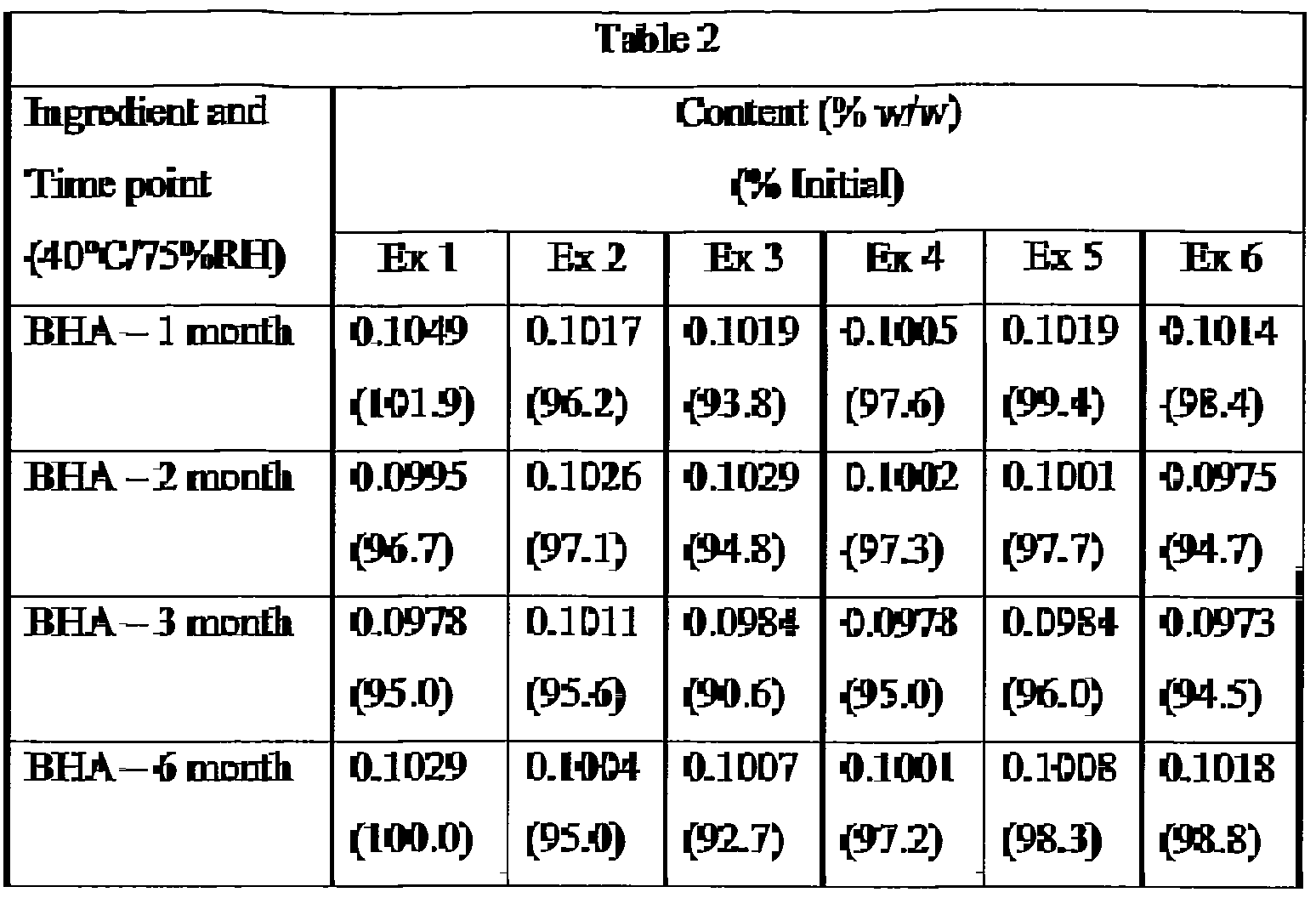
- Example 1-6 The creams of Examples 1-6 were stored at 40°C at 75% relative humidity. At selected time points samples were analyzed for IRM 1, sorbic acid (SA), and BHA content. The results are shown in Table 2 below. The initial values (0 month) are the average of 6 independent determinations (2 samples from each of 3 tubes); the values for the later time points are the average of 2 independent determinations (2 samples from 1 tube). Values are not normalized for weight loss that may have occuned during storage. Test Method 1 was used to determine the IRM 1 content and sorbic acid content. Test Method 2 was used to determine the BHA content.
- Table 3 summarizes topical formulations made in accordance with the present invention in a percentage weight-by- weight basis and a formulation prepared without an antioxidant (CI). The formulations were packaged in glass containers.
- the creams of Examples 7, 8, and CI were stored at 40°C at 75% relative humidity and at 55°C at ambient humidity. At selected time points samples were analyzed using Test Method 1 described above for IRM 1 and sorbic acid content. The results are shown in Table 4 below where each value is for 1 sample from 1 container of cream. Values were not normalized for weight loss that may have occuned during storage.
- Examples 9-18 Table 5 summarizes topical formulations made in accordance with the present invention in a percentage weight-by-weight basis.
- the formulations were packaged in aluminum tubes with an epoxy phenolic lacquer liner.
- the formulations of Examples 9- 18 were stored at 40°C at 75% relative humidity.
- samples were analyzed for IRM 1, sorbic acid (SA), and BHA content.
- the results are shown in Table 6 below where the initial values of IRM and SA are the average of 6 independent determinations (2 samples from each of 3 tubes), the initial BHA values are the average of 3 independent determinations (1 sample from each of 3 tubes), and the values for later time points are the values for one sample from 1 tube. Values are not normalized for weight loss that may have occuned during storage.
- Test Method 1 was used to determine the IRM 1 content and sorbic acid content.
- Test Method 2 was used to determine the BHA content.
- Examples 19-24 Table 7 summarizes topical formulations made in accordance with the present invention in a percentage weight-by-weight basis.
- the formulations were packaged in aluminum tubes with an epoxy phenolic lacquer liner.
- the formulations of Examples 19-24 were stored at 40°C at 75% relative humidity.
- samples were analyzed for IRM 1, sorbic acid (SA), and BHA content.
- the results are shown in Table 8 below where the initial values of IRM SA, and BHA are the average of 3 independent determinations, and the values for later time points are from 1 tube. Values are not normalized for weight loss that may have occuned during storage.
- Test Method 3 was used to determine IRM 1 content.
- Test Method 4 was used to determine SA and BHA content.
- Table 9 summarizes topical formulations made in accordance with the present invention in a percentage weight-by-weight basis. The formulations were packaged in aluminum tubes with an epoxy phenolic lacquer liner.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Dermatology (AREA)
- Dispersion Chemistry (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Virology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Paints Or Removers (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Description
Claims
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES05725623.2T ES2665342T3 (en) | 2004-03-15 | 2005-03-14 | Formulations and methods for immune response modifiers |
| US10/598,824 US20070167479A1 (en) | 2004-03-15 | 2005-03-14 | Immune response modifier formulations and methods |
| JP2007504030A JP4991520B2 (en) | 2004-03-15 | 2005-03-14 | Immune response modulator formulation and method |
| CA2559607A CA2559607C (en) | 2004-03-15 | 2005-03-14 | Immune response modifier formulations and methods |
| AU2005222995A AU2005222995B2 (en) | 2004-03-15 | 2005-03-14 | Immune response modifier formulations and methods |
| EP05725623.2A EP1729768B1 (en) | 2004-03-15 | 2005-03-14 | Immune response modifier formulations and methods |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US55314804P | 2004-03-15 | 2004-03-15 | |
| US60/553,148 | 2004-03-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2005089317A2 true WO2005089317A2 (en) | 2005-09-29 |
| WO2005089317A3 WO2005089317A3 (en) | 2006-07-27 |
Family
ID=34994263
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2005/008576 WO2005089317A2 (en) | 2004-03-15 | 2005-03-14 | Immune response modifier formulations and methods |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20070167479A1 (en) |
| EP (1) | EP1729768B1 (en) |
| JP (1) | JP4991520B2 (en) |
| AU (1) | AU2005222995B2 (en) |
| CA (1) | CA2559607C (en) |
| ES (1) | ES2665342T3 (en) |
| WO (1) | WO2005089317A2 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006071997A2 (en) | 2004-12-30 | 2006-07-06 | 3M Innovative Properties Company | Treatment for cutaneous metastases |
| EP1865957A2 (en) * | 2005-03-14 | 2007-12-19 | Meda AB | Method of treating actinic keratosis |
| US7655672B2 (en) | 2004-12-17 | 2010-02-02 | 3M Innovative Properties Company | Immune response modifier formulations containing oleic acid and methods |
| US8889154B2 (en) | 2005-09-15 | 2014-11-18 | Medicis Pharmaceutical Corporation | Packaging for 1-(2-methylpropyl)-1H-imidazo[4,5-c] quinolin-4-amine-containing formulation |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040265351A1 (en) | 2003-04-10 | 2004-12-30 | Miller Richard L. | Methods and compositions for enhancing immune response |
| CA2536136C (en) | 2003-08-27 | 2012-10-30 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| JP5043435B2 (en) | 2003-10-03 | 2012-10-10 | スリーエム イノベイティブ プロパティズ カンパニー | Alkoxy substituted imidazoquinolines |
| NZ546274A (en) | 2003-10-03 | 2009-12-24 | 3M Innovative Properties Co | Pyrazolopyridines and analags thereof |
| AR046781A1 (en) | 2003-11-25 | 2005-12-21 | 3M Innovative Properties Co | IMIDAZOQUINOLINE DERIVATIVES. PHARMACEUTICAL COMPOSITIONS. |
| US8541438B2 (en) | 2004-06-18 | 2013-09-24 | 3M Innovative Properties Company | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| WO2006074003A2 (en) | 2004-12-30 | 2006-07-13 | 3M Innovative Properties Company | CHIRAL FUSED [1,2]IMIDAZO[4,5-c] RING COMPOUNDS |
| AU2006212765B2 (en) | 2005-02-09 | 2012-02-02 | 3M Innovative Properties Company | Alkyloxy substituted thiazoloquinolines and thiazolonaphthyridines |
| AU2006338521A1 (en) | 2005-02-09 | 2007-10-11 | Coley Pharmaceutical Group, Inc. | Oxime and hydroxylamine substituted thiazolo(4,5-c) ring compounds and methods |
| CA2597446A1 (en) | 2005-02-11 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Substituted imidazoquinolines and imidazonaphthyridines |
| US8178677B2 (en) | 2005-02-23 | 2012-05-15 | 3M Innovative Properties Company | Hydroxyalkyl substituted imidazoquinolines |
| AU2006216799A1 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazonaphthyridines |
| AU2006216686A1 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Method of preferentially inducing the biosynthesis of interferon |
| JP2008531567A (en) | 2005-02-23 | 2008-08-14 | コーリー ファーマシューティカル グループ,インコーポレイテッド | Hydroxyalkyl-substituted imidazoquinoline compounds and methods |
| EA200800782A1 (en) | 2005-09-09 | 2008-08-29 | Коли Фармасьютикал Груп, Инк. | AMIDA AND CARBAMATE DERIVATIVES N- {2- [4-AMINO-2- (ETOXIMETHYL) -1H-IMIDAZOLO [4,5-c] QUINOLIN-1-IL] -1,1-DIMETHYLETHYL} METHANE SULFONAMIDE AND METHODS |
| ZA200803029B (en) | 2005-09-09 | 2009-02-25 | Coley Pharm Group Inc | Amide and carbamate derivatives of alkyl substituted /V-[4-(4-amino-1H-imidazo[4,5-c] quinolin-1-yl)butyl] methane-sulfonamides and methods |
| JP5247458B2 (en) | 2005-11-04 | 2013-07-24 | スリーエム・イノベイティブ・プロパティーズ・カンパニー | Hydroxy and alkoxy substituted 1H-imidazoquinolines and methods |
| WO2007100634A2 (en) | 2006-02-22 | 2007-09-07 | 3M Innovative Properties Company | Immune response modifier conjugates |
| WO2007106854A2 (en) | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| US8178539B2 (en) | 2006-09-06 | 2012-05-15 | 3M Innovative Properties Company | Substituted 3,4,6,7-tetrahydro-5H-1,2a,4a,8-tetraazacyclopenta[cd]phenalenes and methods |
| HUE033901T2 (en) | 2010-08-17 | 2018-01-29 | 3M Innovative Properties Co | Lipidated immune response modifier compound compositions, formulations, and methods |
| BR112013031039B1 (en) | 2011-06-03 | 2020-04-28 | 3M Innovative Properties Co | hydrazine compounds 1h-imidazoquinoline-4-amines, conjugates made from these compounds, composition and pharmaceutical composition comprising said compounds and conjugates, uses thereof and method of manufacturing the conjugate |
| JP6460789B2 (en) | 2011-06-03 | 2019-01-30 | スリーエム イノベイティブ プロパティズ カンパニー | Heterobifunctional linker having polyethylene glycol segment and immune response modulating complex prepared from the linker |
| EP3728255B1 (en) | 2017-12-20 | 2022-01-26 | 3M Innovative Properties Company | Amide substituted imidazo[4,5-c]quinoline compounds with a branched chain linking group for use as an immune response modifier |
| WO2019195802A1 (en) * | 2018-04-05 | 2019-10-10 | Bausch Health Ireland Limited | Polymeric emulsion delivery systems |
| US11891588B2 (en) | 2019-07-31 | 2024-02-06 | Ecolab Usa Inc. | Personal protective equipment free delimer compositions o |
Citations (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4689338A (en) | 1983-11-18 | 1987-08-25 | Riker Laboratories, Inc. | 1H-Imidazo[4,5-c]quinolin-4-amines and antiviral use |
| US4929624A (en) | 1989-03-23 | 1990-05-29 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo(4,5-c)quinolin-4-amines |
| EP0394026A1 (en) | 1989-04-20 | 1990-10-24 | Riker Laboratories, Inc. | Formulation containing an imidazo[4,5-c]quinolin derivative |
| US4988815A (en) | 1989-10-26 | 1991-01-29 | Riker Laboratories, Inc. | 3-Amino or 3-nitro quinoline compounds which are intermediates in preparing 1H-imidazo[4,5-c]quinolines |
| US5037986A (en) | 1989-03-23 | 1991-08-06 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo[4,5-c]quinolin-4-amines |
| US5175296A (en) | 1991-03-01 | 1992-12-29 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-c]quinolin-4-amines and processes for their preparation |
| US5238944A (en) | 1988-12-15 | 1993-08-24 | Riker Laboratories, Inc. | Topical formulations and transdermal delivery systems containing 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine |
| US5266575A (en) | 1991-11-06 | 1993-11-30 | Minnesota Mining And Manufacturing Company | 2-ethyl 1H-imidazo[4,5-ciquinolin-4-amines |
| US5268376A (en) | 1991-09-04 | 1993-12-07 | Minnesota Mining And Manufacturing Company | 1-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5352784A (en) | 1993-07-15 | 1994-10-04 | Minnesota Mining And Manufacturing Company | Fused cycloalkylimidazopyridines |
| US5367076A (en) | 1990-10-05 | 1994-11-22 | Minnesota Mining And Manufacturing Company | Process for imidazo[4,5-C]quinolin-4-amines |
| US5389640A (en) | 1991-03-01 | 1995-02-14 | Minnesota Mining And Manufacturing Company | 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5395937A (en) | 1993-01-29 | 1995-03-07 | Minnesota Mining And Manufacturing Company | Process for preparing quinoline amines |
| US5446153A (en) | 1993-07-15 | 1995-08-29 | Minnesota Mining And Manufacturing Company | Intermediates for imidazo[4,5-c]pyridin-4-amines |
| US5482936A (en) | 1995-01-12 | 1996-01-09 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-C]quinoline amines |
| US5693811A (en) | 1996-06-21 | 1997-12-02 | Minnesota Mining And Manufacturing Company | Process for preparing tetrahdroimidazoquinolinamines |
| US5741908A (en) | 1996-06-21 | 1998-04-21 | Minnesota Mining And Manufacturing Company | Process for reparing imidazoquinolinamines |
| US5756747A (en) | 1989-02-27 | 1998-05-26 | Riker Laboratories, Inc. | 1H-imidazo 4,5-c!quinolin-4-amines |
| US5939090A (en) | 1996-12-03 | 1999-08-17 | 3M Innovative Properties Company | Gel formulations for topical drug delivery |
| US6039969A (en) | 1996-10-25 | 2000-03-21 | 3M Innovative Properties Company | Immune response modifier compounds for treatment of TH2 mediated and related diseases |
| US6069149A (en) | 1997-01-09 | 2000-05-30 | Terumo Kabushiki Kaisha | Amide derivatives and intermediates for the synthesis thereof |
| US6083505A (en) | 1992-04-16 | 2000-07-04 | 3M Innovative Properties Company | 1H-imidazo[4,5-C]quinolin-4-amines as vaccine adjuvants |
| US6110929A (en) | 1998-07-28 | 2000-08-29 | 3M Innovative Properties Company | Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof |
| US6194425B1 (en) | 1997-12-11 | 2001-02-27 | 3M Innovative Properties Company | Imidazonaphthyridines |
| US6245776B1 (en) | 1999-01-08 | 2001-06-12 | 3M Innovative Properties Company | Formulations and methods for treatment of mucosal associated conditions with an immune response modifier |
| US6331539B1 (en) | 1999-06-10 | 2001-12-18 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| US20020016332A1 (en) | 2000-03-30 | 2002-02-07 | Slade Herbert B. | Method for the treatment of dermal lesions caused by envenomation |
| US6376669B1 (en) | 1999-11-05 | 2002-04-23 | 3M Innovative Properties Company | Dye labeled imidazoquinoline compounds |
| US20020055517A1 (en) | 2000-09-15 | 2002-05-09 | 3M Innovative Properties Company | Methods for delaying recurrence of herpes virus symptoms |
| US20020110840A1 (en) | 2000-12-08 | 2002-08-15 | 3M Innovative Properties Company | Screening method for identifying compounds that selectively induce interferon alpha |
| US6451810B1 (en) | 1999-06-10 | 2002-09-17 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US6518265B1 (en) | 1998-08-12 | 2003-02-11 | Hokuriku Seiyaku Co., Ltd. | 1H-imidazopyridine derivatives |
| US6525064B1 (en) | 2000-12-08 | 2003-02-25 | 3M Innovative Properties Company | Sulfonamido substituted imidazopyridines |
| US6541485B1 (en) | 1999-06-10 | 2003-04-01 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US6545017B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Urea substituted imidazopyridines |
| US6545016B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Amide substituted imidazopyridines |
| US6558951B1 (en) | 1999-02-11 | 2003-05-06 | 3M Innovative Properties Company | Maturation of dendritic cells with immune response modifying compounds |
| US6573273B1 (en) | 1999-06-10 | 2003-06-03 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US20030133913A1 (en) | 2001-08-30 | 2003-07-17 | 3M Innovative Properties Company | Methods of maturing plasmacytoid dendritic cells using immune response modifier molecules |
| US20030161797A1 (en) | 2002-02-22 | 2003-08-28 | 3M Innovative Properties Company | Method of reducing and treating UVB-induced immunosuppression |
| US20030199538A1 (en) | 2001-11-29 | 2003-10-23 | 3M Innovative Properties Company | Pharmaceutical formulation comprising an immune response modifier |
| US6656938B2 (en) | 2000-12-08 | 2003-12-02 | 3M Innovative Properties Company | Urea substituted imidazoquinoline ethers |
| US6660747B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| US6660735B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Urea substituted imidazoquinoline ethers |
| US6664265B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| US6664264B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| US6664260B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Heterocyclic ether substituted imidazoquinolines |
| US6667312B2 (en) | 2000-12-08 | 2003-12-23 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| US6677348B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Aryl ether substituted imidazoquinolines |
| US6677347B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamido ether substituted imidazoquinolines |
| US6677349B1 (en) | 2001-12-21 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| US20040014779A1 (en) | 2001-11-16 | 2004-01-22 | 3M Innovative Properties Company | Methods and compositions related to IRM compounds and toll-like recptor pathways |
| US6743920B2 (en) | 2002-05-29 | 2004-06-01 | 3M Innovative Properties Company | Process for imidazo[4,5-c]pyridin-4-amines |
| US6756382B2 (en) | 1999-06-10 | 2004-06-29 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US20040147543A1 (en) | 2002-12-20 | 2004-07-29 | 3M Innovative Properties Company | Aryl substituted imidazoquinolines |
| US20040176367A1 (en) | 2003-03-07 | 2004-09-09 | 3M Innovative Properties Company | 1-Amino 1H-imidazoquinolines |
| US20040175336A1 (en) | 2003-03-04 | 2004-09-09 | 3M Innovative Properties Company | Prophylactic treatment of UV-induced epidermal neoplasia |
| US20040181211A1 (en) | 2003-03-13 | 2004-09-16 | 3M Innovative Properties Company | Method of tattoo removal |
| US20040181130A1 (en) | 2003-03-13 | 2004-09-16 | 3M Innovative Properties Company | Methods for diagnosing skin lesions |
| US20040180919A1 (en) | 2003-03-13 | 2004-09-16 | 3M Innovative Properties Company | Methods of improving skin quality |
| US6797718B2 (en) | 2002-06-07 | 2004-09-28 | 3M Innovative Properties Company | Ether substituted imidazopyridines |
| US20040192585A1 (en) | 2003-03-25 | 2004-09-30 | 3M Innovative Properties Company | Treatment for basal cell carcinoma |
| US6818650B2 (en) | 2002-09-26 | 2004-11-16 | 3M Innovative Properties Company | 1H-imidazo dimers |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US664260A (en) * | 1897-09-29 | 1900-12-18 | William Hamlin | Manufacture of starch. |
| US3906108A (en) * | 1973-10-12 | 1975-09-16 | Johnson & Johnson | Stabilized tretinoin cream emulsion |
| US5721275A (en) * | 1989-06-07 | 1998-02-24 | Bazzano; Gail S. | Slow release vehicles for minimizing skin irritancy of topical compositions |
| SE464167B (en) * | 1989-07-31 | 1991-03-18 | Analytecon Sa | TOPICAL PHARMACEUTICAL PREPARATION OF PODOPHYLLOTOXIN |
| US5079001A (en) * | 1989-11-03 | 1992-01-07 | Ciba-Geigy Corporation | Liquid oral formulation of diclofenac |
| US5583136A (en) * | 1990-01-29 | 1996-12-10 | Johnson & Johnson Consumer Products, Inc. | Retinoid containing skin care compositions containing imidazoles |
| DK0453603T3 (en) * | 1990-04-26 | 1993-11-22 | Com Pharm Arzneimittel Gmbh | Piroxicam-containing pharmaceutical preparations for topical use |
| US5214035A (en) * | 1992-04-16 | 1993-05-25 | Hoechst-Roussel Agri-Vet Company | Thixotropic formulations |
| JP3313148B2 (en) * | 1992-07-13 | 2002-08-12 | 株式会社資生堂 | External preparation for skin |
| ATE365530T1 (en) * | 1992-07-13 | 2007-07-15 | Shiseido Co Ltd | STABILIZED SKIN CARE PRODUCT CONTAINING RETINOL FOR EXTERNAL USE |
| DE4345186C2 (en) * | 1993-12-27 | 1997-08-14 | Galderma Sa | Hydrocortisone 21-acetate-17-propionate containing W / O lotions |
| US6080393A (en) * | 1994-07-09 | 2000-06-27 | Johnson & Johnson Consumer Products, Inc. | Skin care composition comprising a retinoid |
| DE19701018A1 (en) * | 1997-01-14 | 1998-10-15 | Basf Ag | Aqueous preparations and their use |
| US6007846A (en) * | 1997-05-16 | 1999-12-28 | Townley Jewelry, Inc. | Scented body gel having particulate matter in the form of glitter with predetermined shapes |
| US6200605B1 (en) * | 1997-10-30 | 2001-03-13 | Charles E. Day | Antiflatulent composition |
| AU1044500A (en) * | 1998-11-03 | 2000-05-22 | Byk Gulden Lomberg Chemische Fabrik Gmbh | Imidazonaphthyridines |
| US6231849B1 (en) * | 1999-03-25 | 2001-05-15 | George A. Schiller | Simulated seminal fluid |
| US6372791B1 (en) * | 2000-06-29 | 2002-04-16 | Johnson & Johnson Consumer Companies, Inc. | Method of promoting skin cell metabolism |
| JP3685121B2 (en) * | 2001-10-29 | 2005-08-17 | 神鋼鋼線工業株式会社 | Transparent protective tube for outer cable |
| EP1545597B1 (en) * | 2002-08-15 | 2010-11-17 | 3M Innovative Properties Company | Immunostimulatory compositions and methods of stimulating an immune response |
-
2005
- 2005-03-14 AU AU2005222995A patent/AU2005222995B2/en not_active Ceased
- 2005-03-14 EP EP05725623.2A patent/EP1729768B1/en active Active
- 2005-03-14 JP JP2007504030A patent/JP4991520B2/en not_active Expired - Fee Related
- 2005-03-14 ES ES05725623.2T patent/ES2665342T3/en active Active
- 2005-03-14 CA CA2559607A patent/CA2559607C/en not_active Expired - Fee Related
- 2005-03-14 US US10/598,824 patent/US20070167479A1/en not_active Abandoned
- 2005-03-14 WO PCT/US2005/008576 patent/WO2005089317A2/en active Application Filing
Patent Citations (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4689338A (en) | 1983-11-18 | 1987-08-25 | Riker Laboratories, Inc. | 1H-Imidazo[4,5-c]quinolin-4-amines and antiviral use |
| US5238944A (en) | 1988-12-15 | 1993-08-24 | Riker Laboratories, Inc. | Topical formulations and transdermal delivery systems containing 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine |
| US5756747A (en) | 1989-02-27 | 1998-05-26 | Riker Laboratories, Inc. | 1H-imidazo 4,5-c!quinolin-4-amines |
| US4929624A (en) | 1989-03-23 | 1990-05-29 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo(4,5-c)quinolin-4-amines |
| US5037986A (en) | 1989-03-23 | 1991-08-06 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo[4,5-c]quinolin-4-amines |
| EP0394026A1 (en) | 1989-04-20 | 1990-10-24 | Riker Laboratories, Inc. | Formulation containing an imidazo[4,5-c]quinolin derivative |
| US4988815A (en) | 1989-10-26 | 1991-01-29 | Riker Laboratories, Inc. | 3-Amino or 3-nitro quinoline compounds which are intermediates in preparing 1H-imidazo[4,5-c]quinolines |
| US5367076A (en) | 1990-10-05 | 1994-11-22 | Minnesota Mining And Manufacturing Company | Process for imidazo[4,5-C]quinolin-4-amines |
| US5389640A (en) | 1991-03-01 | 1995-02-14 | Minnesota Mining And Manufacturing Company | 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5175296A (en) | 1991-03-01 | 1992-12-29 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-c]quinolin-4-amines and processes for their preparation |
| US5268376A (en) | 1991-09-04 | 1993-12-07 | Minnesota Mining And Manufacturing Company | 1-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5346905A (en) | 1991-09-04 | 1994-09-13 | Minnesota Mining And Manufacturing Company | 1-substituted 1H-imidazo-[4,5-C]quinolin-4-amines |
| US5266575A (en) | 1991-11-06 | 1993-11-30 | Minnesota Mining And Manufacturing Company | 2-ethyl 1H-imidazo[4,5-ciquinolin-4-amines |
| US6083505A (en) | 1992-04-16 | 2000-07-04 | 3M Innovative Properties Company | 1H-imidazo[4,5-C]quinolin-4-amines as vaccine adjuvants |
| US5395937A (en) | 1993-01-29 | 1995-03-07 | Minnesota Mining And Manufacturing Company | Process for preparing quinoline amines |
| US5446153A (en) | 1993-07-15 | 1995-08-29 | Minnesota Mining And Manufacturing Company | Intermediates for imidazo[4,5-c]pyridin-4-amines |
| US5352784A (en) | 1993-07-15 | 1994-10-04 | Minnesota Mining And Manufacturing Company | Fused cycloalkylimidazopyridines |
| US5482936A (en) | 1995-01-12 | 1996-01-09 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-C]quinoline amines |
| US5693811A (en) | 1996-06-21 | 1997-12-02 | Minnesota Mining And Manufacturing Company | Process for preparing tetrahdroimidazoquinolinamines |
| US5741908A (en) | 1996-06-21 | 1998-04-21 | Minnesota Mining And Manufacturing Company | Process for reparing imidazoquinolinamines |
| US6039969A (en) | 1996-10-25 | 2000-03-21 | 3M Innovative Properties Company | Immune response modifier compounds for treatment of TH2 mediated and related diseases |
| US5939090A (en) | 1996-12-03 | 1999-08-17 | 3M Innovative Properties Company | Gel formulations for topical drug delivery |
| US6069149A (en) | 1997-01-09 | 2000-05-30 | Terumo Kabushiki Kaisha | Amide derivatives and intermediates for the synthesis thereof |
| US6194425B1 (en) | 1997-12-11 | 2001-02-27 | 3M Innovative Properties Company | Imidazonaphthyridines |
| US6110929A (en) | 1998-07-28 | 2000-08-29 | 3M Innovative Properties Company | Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof |
| US6518265B1 (en) | 1998-08-12 | 2003-02-11 | Hokuriku Seiyaku Co., Ltd. | 1H-imidazopyridine derivatives |
| US6245776B1 (en) | 1999-01-08 | 2001-06-12 | 3M Innovative Properties Company | Formulations and methods for treatment of mucosal associated conditions with an immune response modifier |
| US6558951B1 (en) | 1999-02-11 | 2003-05-06 | 3M Innovative Properties Company | Maturation of dendritic cells with immune response modifying compounds |
| US6451810B1 (en) | 1999-06-10 | 2002-09-17 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US6756382B2 (en) | 1999-06-10 | 2004-06-29 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US6573273B1 (en) | 1999-06-10 | 2003-06-03 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US6331539B1 (en) | 1999-06-10 | 2001-12-18 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| US6541485B1 (en) | 1999-06-10 | 2003-04-01 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US6376669B1 (en) | 1999-11-05 | 2002-04-23 | 3M Innovative Properties Company | Dye labeled imidazoquinoline compounds |
| US20020016332A1 (en) | 2000-03-30 | 2002-02-07 | Slade Herbert B. | Method for the treatment of dermal lesions caused by envenomation |
| US20020055517A1 (en) | 2000-09-15 | 2002-05-09 | 3M Innovative Properties Company | Methods for delaying recurrence of herpes virus symptoms |
| US6660747B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| US6664265B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| US20020110840A1 (en) | 2000-12-08 | 2002-08-15 | 3M Innovative Properties Company | Screening method for identifying compounds that selectively induce interferon alpha |
| US6683088B2 (en) | 2000-12-08 | 2004-01-27 | 3M Innovative Properties Company | Sulfonamido ether substituted imidazoquinolines |
| US6545017B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Urea substituted imidazopyridines |
| US6525064B1 (en) | 2000-12-08 | 2003-02-25 | 3M Innovative Properties Company | Sulfonamido substituted imidazopyridines |
| US6656938B2 (en) | 2000-12-08 | 2003-12-02 | 3M Innovative Properties Company | Urea substituted imidazoquinoline ethers |
| US6677347B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamido ether substituted imidazoquinolines |
| US6660735B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Urea substituted imidazoquinoline ethers |
| US6545016B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Amide substituted imidazopyridines |
| US6664264B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| US6664260B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Heterocyclic ether substituted imidazoquinolines |
| US6667312B2 (en) | 2000-12-08 | 2003-12-23 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| US6670372B2 (en) | 2000-12-08 | 2003-12-30 | 3M Innovative Properties Company | Aryl ether substituted imidazoquinolines |
| US6677348B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Aryl ether substituted imidazoquinolines |
| US20030133913A1 (en) | 2001-08-30 | 2003-07-17 | 3M Innovative Properties Company | Methods of maturing plasmacytoid dendritic cells using immune response modifier molecules |
| US20040014779A1 (en) | 2001-11-16 | 2004-01-22 | 3M Innovative Properties Company | Methods and compositions related to IRM compounds and toll-like recptor pathways |
| US20030199538A1 (en) | 2001-11-29 | 2003-10-23 | 3M Innovative Properties Company | Pharmaceutical formulation comprising an immune response modifier |
| US6677349B1 (en) | 2001-12-21 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| US20030161797A1 (en) | 2002-02-22 | 2003-08-28 | 3M Innovative Properties Company | Method of reducing and treating UVB-induced immunosuppression |
| US6743920B2 (en) | 2002-05-29 | 2004-06-01 | 3M Innovative Properties Company | Process for imidazo[4,5-c]pyridin-4-amines |
| US6797718B2 (en) | 2002-06-07 | 2004-09-28 | 3M Innovative Properties Company | Ether substituted imidazopyridines |
| US6818650B2 (en) | 2002-09-26 | 2004-11-16 | 3M Innovative Properties Company | 1H-imidazo dimers |
| US20040147543A1 (en) | 2002-12-20 | 2004-07-29 | 3M Innovative Properties Company | Aryl substituted imidazoquinolines |
| US20040175336A1 (en) | 2003-03-04 | 2004-09-09 | 3M Innovative Properties Company | Prophylactic treatment of UV-induced epidermal neoplasia |
| US20040176367A1 (en) | 2003-03-07 | 2004-09-09 | 3M Innovative Properties Company | 1-Amino 1H-imidazoquinolines |
| US20040181211A1 (en) | 2003-03-13 | 2004-09-16 | 3M Innovative Properties Company | Method of tattoo removal |
| US20040181130A1 (en) | 2003-03-13 | 2004-09-16 | 3M Innovative Properties Company | Methods for diagnosing skin lesions |
| US20040180919A1 (en) | 2003-03-13 | 2004-09-16 | 3M Innovative Properties Company | Methods of improving skin quality |
| US20040192585A1 (en) | 2003-03-25 | 2004-09-30 | 3M Innovative Properties Company | Treatment for basal cell carcinoma |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP1729768A4 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7655672B2 (en) | 2004-12-17 | 2010-02-02 | 3M Innovative Properties Company | Immune response modifier formulations containing oleic acid and methods |
| WO2006071997A2 (en) | 2004-12-30 | 2006-07-06 | 3M Innovative Properties Company | Treatment for cutaneous metastases |
| EP2394650A1 (en) | 2004-12-30 | 2011-12-14 | 3M Innovative Properties Co. | Use of resiquimod for the treatment of cutaneous metastases |
| EP1865957A2 (en) * | 2005-03-14 | 2007-12-19 | Meda AB | Method of treating actinic keratosis |
| EP1865957A4 (en) * | 2005-03-14 | 2009-05-06 | Meda Ab | Method of treating actinic keratosis |
| US8889154B2 (en) | 2005-09-15 | 2014-11-18 | Medicis Pharmaceutical Corporation | Packaging for 1-(2-methylpropyl)-1H-imidazo[4,5-c] quinolin-4-amine-containing formulation |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2005089317A3 (en) | 2006-07-27 |
| CA2559607C (en) | 2013-02-19 |
| ES2665342T3 (en) | 2018-04-25 |
| AU2005222995B2 (en) | 2010-08-26 |
| JP2007529537A (en) | 2007-10-25 |
| AU2005222995A1 (en) | 2005-09-29 |
| CA2559607A1 (en) | 2005-09-29 |
| EP1729768B1 (en) | 2018-01-10 |
| EP1729768A2 (en) | 2006-12-13 |
| US20070167479A1 (en) | 2007-07-19 |
| EP1729768A4 (en) | 2013-10-30 |
| JP4991520B2 (en) | 2012-08-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1729768B1 (en) | Immune response modifier formulations and methods | |
| US7906524B2 (en) | Pharmaceutical cream having similar or less levels of imiquimod impurity formation as cream with BHA (comparator) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007167479 Country of ref document: US Ref document number: 10598824 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2559607 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007504030 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005222995 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005725623 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2005222995 Country of ref document: AU Date of ref document: 20050314 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005222995 Country of ref document: AU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005725623 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 10598824 Country of ref document: US |