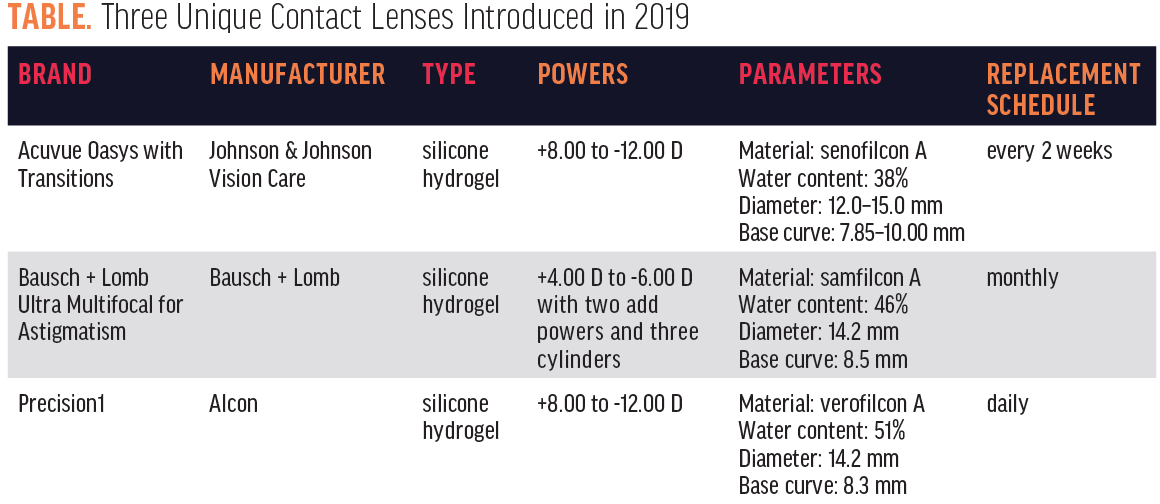
Looking back at the past 12 months, a lot has happened in the world of contact lenses. Many new options were approved by the FDA and/or launched. Manufacturers expanded their lens parameters. One manufacturer reported phase 3 results of an investigational antihistamine-releasing contact lens. Other researchers are working to develop a lens that can release medicine. And there’s more. In this article, I review three unique lenses that were released in 2019 (Table) and offer thoughts on each.
A LIGHT-ADAPTIVE LENS
Voted one of the 50 best inventions of 2018 by Time,1 Acuvue Oasys with Transitions (Johnson & Johnson Vision Care) was launched in April. The lens is built on the backbone of the highly successful Acuvue Oasys lens. What sets it apart is that it contains Transitions Light Intelligent Technology. Like Transitions eyeglasses, the contact lens is designed to quickly and seamlessly respond to changing light conditions while reducing exposure to bright light indoors and out. Available in powers from +8.00 to -12.00 D, and in two base curves, the lens is a unique addition to our offerings.
Not only does Acuvue Oasys with Transitions allow us to protect our patients from harmful UV and blue light; it also presents an opportunity to start a conversation with patients who play outdoor sports or who are light sensitive. Any time one of my patients mentions that he or she is an outdoors enthusiast or struggles with light sensitivity, I mention Acuvue Oasys with Transitions.
Reminding patients about the importance of protection from harmful UV and blue light is a win for optometry, but don’t forget to inform patients that these lenses are not a substitute for sunglasses, as they do not completely cover the eyes and surrounding skin.
A MULTIFOCAL TORIC
Before June, presbyopic patients with astigmatism had to wear custom-ordered contact lenses or compromise their vision results. Bausch + Lomb Ultra Multifocal for Astigmatism (Bausch + Lomb) is the first multifocal toric contact lens available as a standard offering in the eye care professional’s office. The new lens is built on the backbone of Bausch + Lomb Ultra for Astigmatism and Bausch + Lomb Ultra for Presbyopia. It features the Ultra for Astigmatism’s OpticAlign design and Ultra for Presbyopia’s 3-Zone Progressive design.
As a result, the Ultra Multifocal for Astigmatism boasts seamless vision at all distances and a stable fit for consistent results. It also includes Ultra’s MoistureSeal Technology. The lens is available in powers from +4.00 to -6.00 D with two add powers and three cylinder powers. I now look forward to speaking with patients who have both presbyopia and astigmatism, rather than dreading explaining to them that they are not candidates for mainstream soft multifocal contact lenses. No longer do eye care practitioners have to feel limited to offering monovision soft contact lenses for astigmatism with readers over top or masking astigmatism with soft multifocals.
A DAILY IN A NEW MATERIAL
The spherical daily disposable market is already a crowded one, but Precision1 (Alcon) is being marketed as a daily disposable silicone hydrogel lens that is more affordable than Dailies Total1 (Alcon) and more comfortable than Dailies AquaComfort Plus (Alcon) and comparable hydrogel lenses.
Launched in September, Precision1 is made of a new material, verofilcon A. It features class 1 UV blocking and SmartSurface technology, a permanent micro-thin high-performance layer of moisture at the lens surface that helps support a stable tear film to deliver lasting visual performance. The contact lens is available with an 8.3 mm base curve and in powers from +8.00 to -12.00 D.
Although I don’t have this lens in my office yet, I expect that it will be comparable to the clariti 1 day lens (CooperVision).
DON’T BE LEFT BEHIND
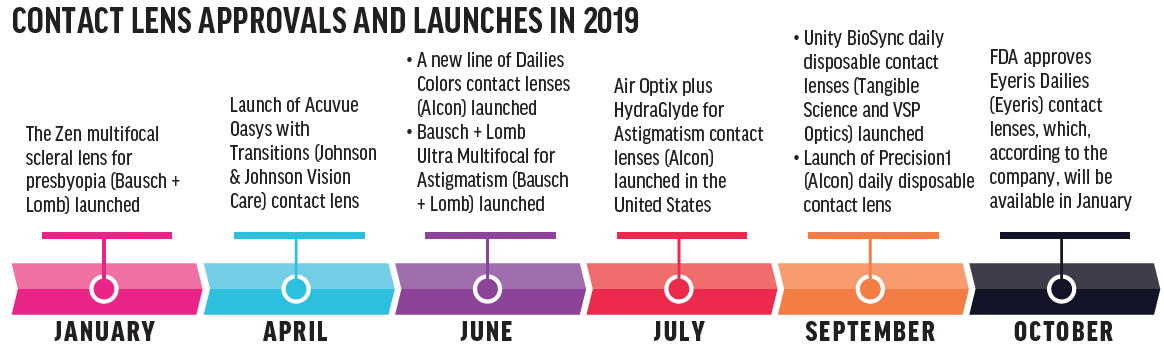
It’s an exciting time to be an eye care practitioner and to fit and prescribe contact lenses. As lens technologies continue to evolve, we’re going to see more new options hit the market (see Contact Lens Approvals and Launches in 2019). In fact, at press time, the FDA approved the MiSight 1 day contact lens (CooperVision), which is indicated to slow the progression of myopia in children between the ages of 8 and 12 years.
With disruptive technologies and direct-to-consumer contact lens companies spreading mixed messages online, it’s in optometrists’ best interest to stay up to date so we can best serve the needs of our patients.
- 1. Best inventions 2018. Time. November 15, 2018. https://time.com/collection/best-inventions-2018/. Accessed October 11, 2019.