WO2011123776A2 - Method for detecting occult blood - Google Patents
Method for detecting occult blood Download PDFInfo
- Publication number
- WO2011123776A2 WO2011123776A2 PCT/US2011/030934 US2011030934W WO2011123776A2 WO 2011123776 A2 WO2011123776 A2 WO 2011123776A2 US 2011030934 W US2011030934 W US 2011030934W WO 2011123776 A2 WO2011123776 A2 WO 2011123776A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- luminol
- reagent
- sodium
- specimen
- chemiluminescent
- Prior art date
Links
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/72—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving blood pigments, e.g. haemoglobin, bilirubin or other porphyrins; involving occult blood
- G01N33/721—Haemoglobin
- G01N33/725—Haemoglobin using peroxidative activity
Definitions
- This invention relates to the field of the detection of occult blood in specimens, particularly in feces.
- Colorectal cancer is common and may be fatal. It is the second-leading cause of death from cancer in the USA. In the absence of effective methods for the prevention of cancer, public health measures have been directed toward early detection.
- the current methods of colorectal cancer screening in the general population include searching for occult blood in the stool, total colonoscopy, flexible sigmoidoscopy, and tests based on abnormal DNA found in fecal specimens.
- Occult bleeding from the gastrointestinal tract may be detected by examination of the excreta for evidence of blood through the use of chemical laboratory techniques.
- the presence of occult blood in the feces is important because it may indicate otherwise asymptomatic
- gastrointestinal neoplasia and may also be helpful in the evaluation of gastrointestinal symptoms in the absence of visible bleeding. Colonoscopy and perhaps examination of the upper gastrointestinal tract with esophagogastroduodenoscopy (EGD) is strongly recommended for anyone with a positive fecal occult blood test (FBOT) on any fecal
- Compliance in non-selected populations has been estimated to be less than 50%, at least partly because the techniques require patients themselves to first select and then smear their stool specimen onto a slide or a test-strip, a task most people find not only loathsome, but also technically difficult.
- Such collection generally involves use of a device that will contact feces deposited in a toilet bowl in the usual manner.
- a device is used to contact the feces, obtain a portion thereof, and is used to streak a sample of the feces on a test strip which will be sent to a laboratory or physician's office for analysis.
- This process is also an unwieldy task, especially if the feces have sunk to the bottom of the toilet bowl.
- Sampling stool from the toilet bowl also introduces error because blood leaches from the fecal surface into the surrounding water which may not be collected for testing, possibly causing a false negative.
- Another testing problem lies with the nature of fecal specimens.
- Fecal specimens are not homogeneous and some portions may have occult blood while others do not.
- a standard FOBT that requires sampling of a small portion is useful, providing the small specimen of stool tested actually contains a representative amount of the blood shed in the gastrointestinal tract by the patient. This implies that a single standard fecal occult blood test that requires sampling may possibly give a false negative result simply because the wrong portion of the fecal specimen was tested. Multiple tests may be required to ensure that any occult bleeding is discovered.
- the intermittent nature of gastrointestinal bleeding confounds the problem since the patient must choose the correct portion of stool to yield an accurate result.
- Luminol preparations have been utilized for the detection of blood at crime scenes or for tracking wounded animals.
- Japanese patent 05256748 discloses use of an ultrasonic vibrator to disrupt the fecal specimen in the toilet bowl. Next, a luminol preparation and peroxides are to be added to the disrupted specimen. Any luminescence which results is then determined with a photodiode sensing section on the vibrator and read with a hand-held meter.
- Japanese patent 05002017 discloses the use of a luminol preparation and hydrogen peroxide. This preparation is to be poured into a commode and any subsequent luminescence is determined with a photodiode or photomultiplier.
- Japanese patent 05273206 A discloses a similar process to Japanese patent 05002017 except that EDTA is to be added prior to adding luminol in an attempt to sequester any trace metals in the water containing the feces to try to prevent false positives due to trace metals like iron or copper in the water supply.
- FIG. 1 shows a flow chart for a method of use of the fecal occult blood detection system of the present invention.
- FIG. 2 shows the proposed reaction pathway for the luminol reagent.
- FIG. 3 shows the results of time vs. chemiluminescence for luminol
- the method employs a reagent comprising an agent selected from the group consisting of purified sodium luminol, purified potassium luminol, cyclic hydrazides and acyclic hydrazides, preferably the sodium or potassium salts of luminol (5-amino-2,3-dihydro 1 ,4-phthalazinedione; o-amino-phthalyhydrazide) and/or the sodium or potassium salts of benzo (ghi)perylene-1 ,2-dicarboxylic acid.
- an agent selected from the group consisting of purified sodium luminol, purified potassium luminol, cyclic hydrazides and acyclic hydrazides, preferably the sodium or potassium salts of luminol (5-amino-2,3-dihydro 1 ,4-phthalazinedione; o-amino-phthalyhydrazide) and/or the sodium or potassium salts of benzo (ghi)perylene
- the method of the invention provides the following advantages as a screening test for fecal occult blood: 1 ) it is simple to use, 2) it is cost effective, 3) it minimizes the percentage of false-negative results (sensitivity), and 4) it has a low incidence of false- positive results (specificity).
- the sensitivity of a fecal occult blood test determines its benefit to the user while the specificity of the test determines its cost effectiveness in terms of the need to screen patients who have positive tests. Since this method requires no fecal specimen sampling, compliance may be increased, thereby overcoming an obstacle found with previous methods.
- the sensitivity and specificity of the invention exceeds that of currently reported "throw-in-the-bowl" tests, thereby meeting another need in the fecal occult blood testing arena.
- the sensitivity exceeds that of the immunochemical methods as well.
- the method of the invention also does not require the use of any device, such as a photodiode or photomultiplier to read the results, thus meeting yet another need for fecal occult blood testing.
- Figure 1 shows a schematic for the method of the current invention for the detection of occult blood.
- a specimen is deposited, usually through direct defecation, into a suitable receptacle, such as a commode bowl, a bedpan or other receptacle.
- a suitable receptacle such as a commode bowl, a bedpan or other receptacle.
- Test reagents are added sequentially to the receptacle containing the specimen, the lights extinguished, and the presence or absence of chemiluminescence is noted. Presence of any chemiluminescence in the receptacle with the specimen is indicative of occult blood.
- the schematic for Figure 1 relates to the use of the method for the present invention with a toilet (commode) bowl as the preferred receptacle for the specimen, but it is understood that other receptacles can be used as stated above.
- a negative control test should be performed in the following manner.
- a sufficient quantity of an oxidizing solution as taught herein is poured into the water of a commode (toilet) bowl. Said quantity of oxidizing solution is of an adequate amount for the approximately 1800 ml_ of water contained in a typical toilet bowl.
- a predetermined quantity of a chemiluminescent reagent, as taught herein is poured into the commode.
- the room light is turned out, and the toilet bowl is observed.
- any neon-like glow observed from the commode water with this procedure indicates residual toilet bowl cleaners or trace metals from the plumbing in the commode water. If a blue glowing light occurs with the use of only the oxidizing solution and the chemiluminescent solution (1 E), the patient is instructed to stop the test, and then is further instructed in 1 F to avoid using toilet bowl cleaners, to remove any cleaning products that may be present in the commode tank, and to flush the commode twice a day over a three day period, after which time, the negative control test should be repeated. If chemiluminescence is again present, the cause is the presence of excess trace metals in the water supply and the test for occult blood should not be performed in this commode.
- the patient must defecate in a bedpan or other receptacle to test for fecal occult blood.
- Approximately one liter of deionized water (enough to cover the fecal specimen) should be poured over the fecal specimen in the receptacle.
- the reagents are added per Step 3 of FIG. 1 as if the test were being performed in a toilet bowl. If an alternate receptacle is utilized, the negative and positive control steps should be omitted.
- the control is considered to be acceptably negative (1 D), and the patient is instructed to flush the toilet twice to remove the reagents from the toilet bowl before proceeding to Testing Procedure Step 2 - Positive Control.
- Any neon-like blue glowing light that may occur when flushing the commode in the dark is to be ignored. Even though a negative control test can be completely negative, a brilliant, blue glowing light may be produced when the commode is flushed. This is due to trace metals from the toilet bowl tank mixing with the reagents and does not represent occult blood in the fecal specimen.
- Step 2 Positive Control. If the negative control test is satisfactorily negative, a positive control test is preferably performed to illustrate to the user how a positive test should appear.
- the positive control may be oxidizing compounds like hypochlorite, iodine, or iodophors.
- Dried beef blood functions well as a positive control, as does purified human hemoglobin that has been tested free of HIV and hepatitis. Hemin or Sodium and potassium salts of Hemin (Frontier Scientific, Inc. in, Logan, Utah, USA) are the positive controls of choice.
- an adequate quantity of an oxidizing solution (2A) is added to the commode water.
- a positive control agent for example, one drop of a Hemin solution from Porphyrin Products, Inc. containing 150 mg hemin/mL dissolved in ethanol
- the chemiluminescent or chemifluorescent compound of 2C is sprayed or poured into the toilet bowl water and the room is darkened (2D) prior to reading the test.
- the chemiluminescence or chemifluorescence detected with the naked eye in the darkened room is noted by the user.
- Step 3 Patient Sample.
- the patient defecates or micturates (3A) into the toilet bowl.
- 3B an adequate quantity of the oxidizing solution is added to the toilet bowl water.
- an adequate volume of the oxidizing solution is added to the toilet bowl water.
- chemiluminescent or chemifluorescent compound 3C
- chemiluminescent or chemifluorescent compound 3C
- the fecal or urine sample contains blood or its degradation products like heme, hemoglobin, protoporphyrin IX, or iron, there is an immediate dramatic production of a neon-like blue light visible to the naked eye in the darkened room, which may be seen without the use of photodiodes or photomultipliers.
- the amount of light production depends on the amount of heme, hemoglobin,
- protoporphyrin IX or iron in the toilet bowl water. If any amount of neon-like glowing blue light is seen, the test is positive (3F) for occult blood and the patient is instructed to contact a physician regarding follow-up testing, such as a colonoscopy. (3G). If the sample in the toilet bowl water does not contain heme, hemoglobin, protoporphyrin IX, or iron, there will be no light visible and the test is considered negative for occult blood (3E).
- the preferred chemiluminescent and/or chemi-fluorescent compound is sodium luminol.
- this invention requires the application of an oxidizing compound either just prior or subsequent to micturition or defecation, followed by application of a chemiluminescent or chemifluorescent compound.
- the oxidizing compound and chemiluminescent or chemifluorescent components may be mixed together just prior to application to the urine and/or fecal specimen.
- the reaction may also be achieved by simply pouring the chemicals into the device containing the specimen if there is enough water in the device..
- One mode of applying the required chemicals is with a bottle fitted with a spray nozzle.
- the volume delivered by the spray nozzle should be such that approximately five to ten actuations of the spray nozzle are needed to apply an adequate quantity of each chemical for each test.
- a preferred method is to simply pour an adequate volume of hydrogen peroxide containing a chelator into the container holding the specimen followed by pouring an adequate volume of the chemiluminescent reagent.
- the test for occult blood of the current invention may also be used on aspirates of gastric juice obtained via naso-gastric tubes.
- the hydrogen peroxide and sodium luminol are poured into the receptacle containing the specimen, the light is turned off and any chemiluminescence is easily determined.
- the test for occult blood of the current invention may also be used on sputum samples.
- a patient would take an inhalation treatment with hypertonic saline (three percent sodium chloride) administered via a regular nebulizer or ultrasonic nebulizer.
- the hypertonic saline would induce water into the bronchial tubes and induce sputum production.
- the sputum is saved in a sterile cup.
- the hydrogen peroxide and sodium Luminol are poured into the cup.
- the light is turned off.
- the production of chemiluminescence indicates occult blood in the sputum.
- the patient should be referred for bronchoscopy. This may lead to an earlier detection of bronchial carcinoma.
- the test for occult blood of the current invention may also be used on urine samples.
- the urine could be tested in the commode water as one would test for occult blood on fecal samples.
- a one to two ounce urine specimen could have the oxidizing agent and Sodium Luminol added to the sterile urine cup. The light is turned off. The production of chemiluminescent indicates occult blood in the urine.
- the patient will require an intravenous pyelogram and perhaps cystoscopy.
- the volume of the reagents necessary for the reaction optimization will vary depending on the volume of the receptacle holding the specimen.
- the preferred range of volume of the oxidizing reagent for a standard commode bowl is 10-40 ml_ of the 5 % hydrogen peroxide - chelator solution, with 30 ml_ being most preferred.
- the preferred range of volume of the chemiluminescent reagent for a standard commode bowl is 10- 50 ml_ of the reagent containing 2 mg/mL purified luminol in 75 mg/mL sodium
- the fecal (stool) specimen contains peroxidases from plants, or catalases then there may be very minuscule light output from a multitude sites on the stool surface. Blood produces a dramatic neon-like blue light output which is sustained and easily seen in a dark room, whereas peroxidases from other sources produce only minuscule, if any, light output.
- the light output from heme, hemoglobin, protoporphyrin IX, or iron is both dramatic and prolonged. The light output may last as long as ten minutes. The light output may also be reproduced by a second application of the chemiluminescent reagent disclosed herein.
- the method of the invention overcomes a deficiency in other tests for fecal occult blood, i.e., guaiac, which are accompanied by an unacceptably large number of false positives due to dietary peroxidases which are common in onion, bell peppers and radishes, for example.
- Human hemoglobin is a pseudo-peroxidase.
- the false- positives caused by dietary peroxidases are because the ideal pH for performing guaiac tests and detecting dietary sources of peroxidases overlap.
- an oxidizing reagent is added to the receptacle holding the specimen.
- An oxidizing agent is used to lyse any red blood cells present in the specimen in order to release the hemoglobin.
- the oxidizing compound serves as a catalyst for luminol.
- the oxidizing reagent is selected from inorganic peroxides, organic peroxides, and mixtures thereof.
- the oxidizing reagent must further comprise a chelator to bind metal ions which may be present in the toilet water either before or after deposition of the specimen.
- the same oxidizing reagent is also used in the negative and positive control tests in steps 1A and 2A.
- inorganic peroxides which may be used are hydrogen peroxide, sodium peroxycarbonate, and sodium perborate or mixtures thereof.
- Preferred inorganic peroxides are hydrogen peroxide, sodium peroxycarbonate and sodium perborate.
- the preferred concentration is between three and five percent. Three percent hydrogen peroxide is most preferred.
- peroxides may influence the choice of chelator utilized as is explained further below.
- Organic peroxides which may be used include diacyl peroxides, ketone peroxides, peroxydicarbonates, peroxyesters, dialkyl peroxides, hydroperoxides or peroxyketals. Examples of preferred organic peroxides are cumene hydroperoxide, benzoyl peroxide or t-butyl hydroperoxide.
- the oxidizing compound preferably is kept in a separate container from the cyclic hydrazide as mixing the solutions may reduce the shelf life of the hydrazide.
- the oxidizing compound may be organic peroxides, inorganic peroxides, or mixtures of the two.
- the peroxide compound may be added as a dry solid, for example,
- peroxycarbonate granules but the preferred embodiment is a liquid containing a trace metal ion chelator.
- This liquid may be either poured or sprayed into the receptacle for receiving the human excrement.
- the inorganic peroxide, organic peroxide or mixture of the two may be added to the water in the toilet bowl either before or after defecation or urination. If the oxidizer is added before the specimen, then following defecation or urination, additional peroxide compound may be applied to the surface of the water.
- a preferred means of application is for the reagent to be poured into the commode water.
- the simplest oxidizing solution that performs well in the novel assay of this invention is hydrogen peroxide. Over-the-counter three percent (3%) hydrogen peroxide functions well as an oxidizer.
- a trace metal chelator must be added to prevent false-positive reactions in the toilet water.
- Hydrogen peroxide must not be used with EDTA in the context of a luminol assay for the detection of blood.
- sodium peroxycarbonate and sodium perborate can be used in conjunction with EDTA.
- An acceptable chelator should have a pH in the range of 4 -7, preferably near neutral, and be compatible with the oxidizer component of the oxidizing reagent.
- a simple test such as follows, may be utilized.
- the proposed chelator should be mixed with the oxidizer of choice and the mixture capped.
- the oxidizer levels are determined using any suitable method for detection of the oxidizer.
- levels are determined using any suitable method for the determination of hydrogen peroxide.
- Such methods may include, inter alia, titration, spectrophotometry, fluorescence, chemiluminescence, electrochemical or any other method suitable for detection of hydrogen peroxide.
- There are also commercial kits available for hydrogen peroxide detection which may be employed. If no substantial change has occurred in hydrogen peroxide levels, the chelator may be used in the method of the present invention.
- a commercially available chelator compatible with hydrogen peroxide is a phosphoric acid-based chelator sold under the tradename DEQUEST FS 0520 as a solution with excellent transition metals chelation properties. (Solutia, Inc., St. Louis, Missouri). This product has a pH of 5.0 and is capable of binding the trace metals in water. The preferred concentration of Dequest FS 0520 is one hundred cc per liter of 3% hydrogen peroxide.
- a reagent comprising a chemiluminescent agent is added to the receptacle containing the specimen.
- the chemiluminescent agent is selected from the purified sodium or potassium salts of luminol (5-amino-2,3-dihydro 1 ,4- phthalazinedione; o-amino-phthalyhydrazide) and/or the sodium or potassium salts of benzo (ghi) perylene-1 ,2-dicarboxylic acid.
- chemiluminescence produced by the occult blood may be detected by the naked eye in the dark without the aid of instrumentation.
- the same chemiluminescent reagent is also used in the negative and positive controls in steps 1 B and 2C.
- chemiluminescence may also be achieved with cyclic or acyclic hydrazides.
- cyclic hydrazides analogs with substituents on the nonheterocyclic ring are easily prepared, but any substitution of the heterocyclic ring renders the compounds non-chemiluminescent.
- Cyclic hydrazide compounds which are up to 150% more efficient in chemiluminescence than luminol, such as benzo (ghi) perylene-1 ,2-dicarboxylic acid hydrazide, can be prepared according to the method of Wei and White. Tetrahdron Letters: Volume 39: 3559 (1971 ) by C. C. Wei and E. H.
- Non-interfering substances which can augment the chemiluminescence are contemplated, but the sensitivity should be sufficient as to not require augmenting the chemiluminescence of the luminol.
- An example of a non-interfering substance would be fluorscein.
- Chemiluminescence is the emission of light (424 nm wave length) from chemical reactions at ordinary temperatures.
- One example of chemiluminescence is the oxidation of luminol (5-amino-2,3-dihydro 1 ,4-phthalazinedione; o-amino- phthalylhydrazide), to produce an intense neon-like blue light.
- Luminol exhibits an intense neon-like blue chemiluminescence by oxidation in an alkaline solution.
- Luminol reacts with animal blood as well as with human blood.
- the advantage of luminol for the detection of blood is its extreme sensitivity. Hematin can be detected in a dilution of 1 :1 ,000,000 or 10 "4 micrograms of crystalline hemoglobin. The duration of the luminescence depends upon the amount of blood present.
- luminol is used in forensic science for the detection of blood at crime scenes. Analytical chemists have also used luminol for the detection of trace amounts of iron, cobalt, copper and chromium (III). Free radical generation from erythrocytes or whole blood has been monitored continuously by luminol-amplified chemiluminescence.
- reaction path shown in FIG. 2 is proposed as an explanation for the chemiluminescence of luminol at an alkaline pH.
- the ambient light is eliminated, and the specimen may be observed with the unaided eye for the presence or absence of a neon-like chemiluminescent blue light.
- chemiluminescence production of a blue light is indicative of blood.
- the light emission produced with the purified luminol reagent according to the method of the invention is as much as one hundred fold greater than the light emission of unpurified luminol.
- the reaction of one drop of blood in a flask containing 1800 cc of water demonstrates that purified Luminol can detect 5 nanograms of hemoglobin per ml_.
- the reaction product can be easily seen with normal vision without the aid of photodiodes or photomultipliers
- An appropriate purity of luminol salt must be utilized. If the luminol starting material has contaminants, then a procedure for purifying luminol must be used. A procedure for the purification of luminol is provided herein. Other purification methods may also be effective. The level of purity can be tested by determining if a known sample comprising a drop of blood oxidized with 3 ml_ of hydrogen peroxide can be detected as stated above with addition of 5 ml_ of an aqueous solution of sodium luminol (2 mg/mL sodium luminol; 75 mg/mL sodium hydroxide) to said oxidized blood with the chemiluminescence visible to the unaided eye.
- the luminol product has a starting purity of >99% with crystals that are cream to white in color, it may be used in the method of invention without further purification providing the chemiluminescence produced is at least 75% of the glow intensity seen with the purified luminol.
- Luminol crystals with >99% purity demonstrating acceptable chemiluminescence as described herein may be acquired from Gold Biotechnology of St. Louis, MO.
- the luminol does not perform to the standard indicated above, it may be purified by the following method.
- crude luminol is first converted to sodium luminol.
- Crude luminol is dissolved to near saturation in 5% weight/volume room temperature sodium hydroxide (i.e. 100 mg/mL), suction filtered through a 0.22 micron membrane (Millipore), and then crystallized at 0°C for four hours.
- the sodium luminol precipitates out leaving a supernatant that is solid black.
- the precipitated crystals are filtered on a Whatman GFA disk, then dissolved in the minimum volume of 5% sodium hydroxide and re-crystallized at 0°C for 18 hours.
- An alternative method of purifying luminol is as follows. One gram of crude luminol is added to 50 mL of deionized water. Seven mL of 1 Molar sodium hydroxide is added to the solution, followed by the drop-wise addition of 1 mL of concentrated hydrochloric acid (37%). The hydrochloric acid brings the pH back to 7.00. During the addition of hydrochloric acid a white precipitate appears. This is purified luminol. The supernatant is removed by aspiration and the precipitate washed with deionized water. The white precipitate is warmed in an oven to evaporate any remaining water. Chemiluminescent Reagent - Preparation of Preferred Luminol Reagent
- a chemiluminescent reagent comprising a basic solution of purified sodium luminol is prepared by solubilizing purified sodium luminol in 75 mg/mL of sodium hydroxide to make a solution with a concentration of from about 0.01 to 5 mg/mL sodium luminol.
- the preferred concentration of the chemiluminescent reagent is about 2.0 mg/mL sodium luminol since above this concentration there is only a slight increase in light output.
- the near zenith of chemiluminescence of purified sodium luminol occurs with a concentration of 2 mg/mL.
- the concentration of sodium hydroxide used in the luminol solution should be sufficient to raise the water in the testing system to a pH of greater than 10, and greater or lesser than 75 mg/mL may be required to achieve that pH.
- the most preferred concentration of sodium hydroxide is 75 mg/mL, which is adequate to raise the pH of the water to the optimum pH of 12.4.
- FIG. 3 shows chemiluminescence vs. time of several luminol concentrations at specified pH values.
- the solution can be Q.S. to pH 12.4 with concentrated hydrochloric acid, per Table II below.
- the average volume of water in a toilet bowl is about 1800 mL.
- Addition of one ounce (30 mL) of the chemiluminescent reagent containing 2 mg/mL sodium luminol in 75 mg/mL of sodium hydroxide will raise the pH of the toilet water to about 12.4. This is necessary to achieve optimal light output allowing the chemiluminescence to be seen with the naked eye in a darkened room without the need of instrumentation.
- the optimal method for detecting occult blood is to perform the test with a pH greater than 10.0, and preferably at a pH of near 12. 4. At this pH, most, if not all, plant peroxidases will be inactivated allowing maximal chemiluminescence to occur and elimination of the false positives caused by plant peroxidases seen with previous FOBT performed at pH 9.0 or less. By raising the pH to greater than 12, hydrogen peroxide is converted to perhydroxyl ions. This is the preferred species of hydrogen peroxide for the oxidation of luminol.
- the pH requirement to reduce or eliminate false-positives due to dietary peroxidases, i.e. > 10 and preferably at pH 12.4, is critical with sodium luminol, which point has not been recognized prior to this invention.
- Tables I and II below provide preferred ingredient ratios for the sodium luminol reagent.
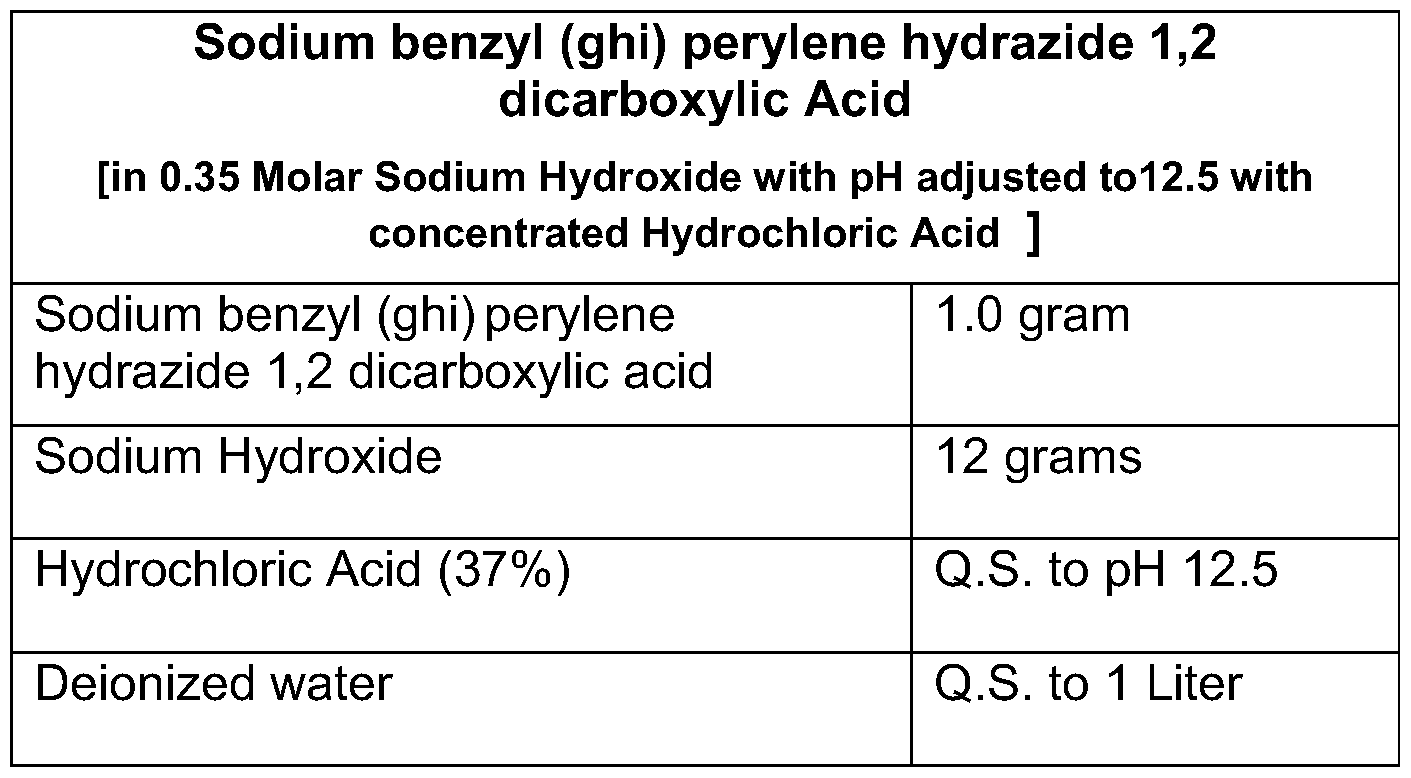
- An alternative preferred chemiluminescent reagent is disodium benzyl (ghi) perylene hydrazide 1 ,2 dicarboxylic acid.
- Formulations for the alternative reagent of the present invention are given in Tables III and IV below. Table III
- a solution of 5% hydrogen peroxide was poured into a two-ounce bottle fitted with a Calmar ® SSA 0.025 spray nozzle (Calamar, Lee's Summit, MO).
- the hydrogen peroxide solution was prepared by diluting a 50% hydrogen peroxide (Food Grade - Solvay Interox) solution one to ten with deionized water.
- An aqueous sodium luminol solution (2.0 mg/mL sodium luminol in 2 Molar sodium hydroxide) was also poured into a two ounce bottle fitted with a Calmar ® SSA 0.025 spray nozzle.
- Turnips, radishes, carrots, apples, horseradish sauce, cantaloupe, watermelon, and celery were purchased from a grocery store. Each vegetable was ground into a slurry with a blender. For each vegetable tested, two ounces of hydrogen peroxide was poured into the commode water, and then the vegetable slurry was added. An aqueous solution of sodium luminol (1 mg/mL sodium luminol; 14 mg/mL sodium hydroxide) was sprayed into the commode water. The bathroom light was then turned off and the water was observed for the blue glow of chemiluminescence.
- Commode water will test negative for chemiluminescence when the oxidizing solution containing chelators and then the sodium luminol/sodium hydroxide solution are sprayed on the water's surface unless the water has been contaminated by toilet bowl cleaners. It has been observed that some commodes will produce a brilliant, glowing blue light during drainage of the commode water during flushing. This is thought to occur due to trace metal contamination from the toilet bowl tank.
- Commodes that have been disinfected with hypochlorites may produce a false positive reaction.
- the reaction with hypochlorites is intense, but brief, lasting less than five seconds. Therefore, false positives from toilet bowl cleaners containing
- hypochlorites can be determined by the distribution, intensity, and length of reaction.
- EXAMPLE 8 DETECTION OF POLYPS IN A 70 YEAR OLD MAN
- guaiac-based test cards (HEMOCCULT®) were obtained and were also numbered consecutively one through five. One cc of fluid from each cup was used to thoroughly soak the cards. These were allowed to air dry. The developer was then used per the package directions.
- the assay of the novel chemiluminescent invention was performed by adding 3 cc of 3% hydrogen peroxide to each cup and mixing. In a dark room, 0.6 cc of purified sodium luminol solution was added to each cup. The results are as follows:
- the novel invention is at least 200,000 times more sensitive for the detection of hemoglobin than the commercially available guaiac test kits This is important since adenomatous polyps less than 2.5 cm in diameter only lose small amounts of blood, i.e., 1 to 2 cc per day.
- the commercially available HEMOCCULT® test (Beckman-Colter) requires 10 cc of blood in a fecal specimen in order to provide correct results about 50% of the time.
- the procedure of the invention can detect hemoglobin at a concentration of 5 nanograms per mL and may be able to detect concentrations in excess of 1 nanograms per mL.
- the novel FOBT was performed on patients scheduled for elective colonoscopy at the Gl Center in Overland Park, Kansas. Volunteers signed informed consent to perform FOBT with the chemiluminescence test of the present invention as well as a commercially available fecal immunochemistry test kit (FIT) (InSure ® brand, Enterix, Inc, Edison, N.J.). The FDA pre-approved product instructions for experimental use of the chemiluminescence test of the invention, and the patients were provided with these instructions. The patients were required to complete an exit questionnaire to ascertain that they understood the instructions. A diet free of red meat, ascorbic acid, iron supplements and vitamins was recommended for three days prior to any stool testing and through the testing period. Volunteers were instructed follow the procedure for observing the negative control before proceeding with the test samples.
- FIT fecal immunochemistry test kit
- the chemiluminescence test kit was positive in eight cases.
- the commercial fecal immunochemistry test (FIT) kit was negative in all thirty of the volunteers. Results are presented in Tables VI and VII below.
- chemiluminescence test detected several adenomatous polyps.
- the smallest adenomatous polyp detected was 6 mm.
- the other four patients' adenomatous polyps were all 10 mm. All adenomatous polyps were removed with a snare and were free of metaplasia or dysplasia.
- the chemiluminescence test was positive when polyps were located in the cecum, hepatic flexure, descending colon and rectal area. The chemiluminescence test did not detect any hemorrhage from four patients who had 3 mm sessile polyps.
- the chemiluminescence test has the ability to detect premalignant lesions in all locations with greater sensitivity than the immunochemical assay and with a high rate of specificity.
- the 6 mm adenomatous polyp was positive on all three bowel movements. If the chemiluminescent test can detect adenomatous polyps where bleeding from a 1 cm polyp is estimated to be 1 .2 cc per day then it should routinely detect a mucosal adenocarcinoma where the bleeding is estimated to be about 5 cc per day.
- a standard guaiac test such as Hemoccult®, blood loss of approximately 10 cc per day is needed for the test to correctly detect bleeding in 50% of the samples.
- Phenalphthalin to detect plant peroxidases in the specimen
- An additional step may be included in the method of the invention utilizing a chromogenic composition which will change colors in the presence of plant
- This chromogenic composition would also change colors in the presence of occult blood following the addition of hydrogen peroxide to lyse the red blood cells. The addition of this step would further reduce the incidence of false positives by identifying samples that contain active plant peroxidases, the presence of which could lead to false positive test results.
- the preferred chromogenic composition comprises an aqueous basic solution of phenolphthalin.
- the sodium salt of phenolphthalin is colorless in deoxygenated alkaline solution, and is readily oxidized by minute quantities of blood in the presence of hydrogen peroxide to phenolphthalein which gives a deep hot pink color in alkaline solution. If plant peroxidases are present in the fecal specimen, the colorless phenolphthalin will oxidize to phenolphthalein producing a hot pink color which is visible to the unaided eye in ambient room lighting. Following the addition of hydrogen peroxide, occult blood will also produce a bright pink glow. If occult blood or plant peroxidases are not present, the composition will remain clear.
- the feces is deposited in the toilet bowl receptacle, an alkaline solution containing approximately 2 mg/mL of phenolphthalin in 0.25 moles/L sodium or potassium hydroxide is added to the receptacle containing the specimen, and the receptacle is observed for 30-45 seconds. If a color change to hot pink is visible, plant peroxidases are present in the sample and the test should be discontinued. Appropriate dietary recommendations should be implemented to reduce these peroxidases before retesting.
- reagents appropriate for a test procedure can be supplied in a kit comprising a container for oxidizing agent, a container of
- the kit preferably further comprises containers of reagent(s) which test for interfering substances in the toilet bowl and/or peroxidases as described above.
Abstract
A novel method for detecting occult blood in feces, urine, gastric juices and sputum is herein disclosed, representing an improvement in occult blood testing, particularly of the type which is readily useable by a lay person in a "self-testing" format. The method is suitable for application to a specimen predeposited in a testing zone, for example a toilet bowl. The need for specimen manipulation is eliminated. The method comprises application of an oxidizing agent preferably containing a metal chelator to the specimen of interest followed by application of a chemiluminescent or chemifluorescent compound. The ambient light is then eliminated allowing the chemiluminescent or chemifluorescent glow to be observed with the unaided eye. The chemiluminescent or chemifluorescent compounds of the invention are selected from purified sodium or potassium luminol, purified sodium or potassium benzyl (ghi) perylene hydrazide 1,2 dicarboxylic acid at a pH greater than 9.0. Using these sodium or potassium salts of hydrazides produces chemiluminescence with occult blood of such intensity that the results may be read with the unaided eye, thus eliminating the need for detection equipment.
Description
METHOD FOR DETECTING OCCULT BLOOD
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Patent Application No. 61/320,038 filed 1 April 2010.
STATEMENT REGARDING FEDERALLY SPOSORED RESEARCH OR
DEVELOPMENT
[0002] Not applicable
TECHNICAL FIELD OF INVENTION
[0003] This invention relates to the field of the detection of occult blood in specimens, particularly in feces.
BACKGROUND OF THE INVENTION
[0004] Colorectal cancer is common and may be fatal. It is the second-leading cause of death from cancer in the USA. In the absence of effective methods for the prevention of cancer, public health measures have been directed toward early detection. The current methods of colorectal cancer screening in the general population include searching for occult blood in the stool, total colonoscopy, flexible sigmoidoscopy, and tests based on abnormal DNA found in fecal specimens.
[0005] There is considerable evidence that screening for fecal blood in asymptomatic individuals who are at average risk can detect cancer at an early and curable stage, resulting in a reduction in mortality. Occult bleeding is loss of blood into the
genitourinary or gastrointestinal tract that is not apparent to the patient or physician either by observation of the excreta or by physical examination. Occult bleeding from the gastrointestinal tract may be detected by examination of the excreta for evidence of blood through the use of chemical laboratory techniques. The presence of occult blood in the feces is important because it may indicate otherwise asymptomatic
gastrointestinal neoplasia and may also be helpful in the evaluation of gastrointestinal symptoms in the absence of visible bleeding. Colonoscopy and perhaps examination of the upper gastrointestinal tract with esophagogastroduodenoscopy (EGD) is strongly
recommended for anyone with a positive fecal occult blood test (FBOT) on any fecal
[0006] Currently, the first step in most screening processes is procurement of a fecal specimen for testing. Patient compliance with screening has been severely
compromised by requiring patients to collect their own fecal specimens. Compliance in non-selected populations has been estimated to be less than 50%, at least partly because the techniques require patients themselves to first select and then smear their stool specimen onto a slide or a test-strip, a task most people find not only loathsome, but also technically difficult.
[0007] Such collection generally involves use of a device that will contact feces deposited in a toilet bowl in the usual manner. A device is used to contact the feces, obtain a portion thereof, and is used to streak a sample of the feces on a test strip which will be sent to a laboratory or physician's office for analysis. This process is also an unwieldy task, especially if the feces have sunk to the bottom of the toilet bowl. Sampling stool from the toilet bowl also introduces error because blood leaches from the fecal surface into the surrounding water which may not be collected for testing, possibly causing a false negative. Another testing problem lies with the nature of fecal specimens. Fecal specimens are not homogeneous and some portions may have occult blood while others do not. A standard FOBT that requires sampling of a small portion is useful, providing the small specimen of stool tested actually contains a representative amount of the blood shed in the gastrointestinal tract by the patient. This implies that a single standard fecal occult blood test that requires sampling may possibly give a false negative result simply because the wrong portion of the fecal specimen was tested. Multiple tests may be required to ensure that any occult bleeding is discovered. In addition, the intermittent nature of gastrointestinal bleeding confounds the problem since the patient must choose the correct portion of stool to yield an accurate result.
[0008] The sensitivity of most FOBT techniques is less than 50% for curable neoplasm. Furthermore, factors such as medications, multiple dietary constituents, variability in fecal hydration, and storage of assay materials commonly produce erroneous results.
[0009] "Throw-in-the-bowl' tests using chromagens for occult blood have been proposed to circumvent the requirement of sampling the fecal specimen. ColoCare by
Helena Laboratories and EZ Detect by BioMedica Inc. are marketed products using the "Throw-in-the-bowl" technique for detecting occult blood. Each of these tests uses guaiac as the reagent for detecting occult blood.
[0010] Luminol preparations have been utilized for the detection of blood at crime scenes or for tracking wounded animals.
[0011] Japanese patent 05256748 discloses use of an ultrasonic vibrator to disrupt the fecal specimen in the toilet bowl. Next, a luminol preparation and peroxides are to be added to the disrupted specimen. Any luminescence which results is then determined with a photodiode sensing section on the vibrator and read with a hand-held meter.
[0012] Japanese patent 05002017 discloses the use of a luminol preparation and hydrogen peroxide. This preparation is to be poured into a commode and any subsequent luminescence is determined with a photodiode or photomultiplier.
[0013] Japanese patent 05273206 A discloses a similar process to Japanese patent 05002017 except that EDTA is to be added prior to adding luminol in an attempt to sequester any trace metals in the water containing the feces to try to prevent false positives due to trace metals like iron or copper in the water supply.
[0014] Requiring use of an electronic device or devices to accomplish testing adds a level of complexity, increasing the cost and the potential for error. It is believed that that EDTA is not compatible with hydrogen peroxide and converts the hydrogen peroxide to water, calling into question the reported technique for utilizing EDTA for sequestering trace metals.
[0015] Thus, there is a continuing need for improved "throw in the bowl tests" for fecal occult blood.
BRIEF DESCRIPTION OF DRAWINGS
[0016] FIG. 1 shows a flow chart for a method of use of the fecal occult blood detection system of the present invention.
[0017] FIG. 2 shows the proposed reaction pathway for the luminol reagent.
[0018] FIG. 3 shows the results of time vs. chemiluminescence for luminol
concentrations at specified pH values at 424 nm.
DETAILED DESCRIPTION
[0019] An in situ method for the detection of occult blood in fecal specimens, urine specimens, gastric juice, and sputum samples is herein disclosed which does not require the use of sensors, photodiodes, or photomultipliers to detect
chemiluminescence resulting from of a reaction between said occult blood and a reagent. Further, the method does not require manipulation of the sample with a vibrator, swab, stick or the like. The method employs a reagent comprising an agent selected from the group consisting of purified sodium luminol, purified potassium luminol, cyclic hydrazides and acyclic hydrazides, preferably the sodium or potassium salts of luminol (5-amino-2,3-dihydro 1 ,4-phthalazinedione; o-amino-phthalyhydrazide) and/or the sodium or potassium salts of benzo (ghi)perylene-1 ,2-dicarboxylic acid.
[0020] The method of the invention provides the following advantages as a screening test for fecal occult blood: 1 ) it is simple to use, 2) it is cost effective, 3) it minimizes the percentage of false-negative results (sensitivity), and 4) it has a low incidence of false- positive results (specificity). The sensitivity of a fecal occult blood test determines its benefit to the user while the specificity of the test determines its cost effectiveness in terms of the need to screen patients who have positive tests. Since this method requires no fecal specimen sampling, compliance may be increased, thereby overcoming an obstacle found with previous methods. The sensitivity and specificity of the invention exceeds that of currently reported "throw-in-the-bowl" tests, thereby meeting another need in the fecal occult blood testing arena. The sensitivity exceeds that of the immunochemical methods as well. The method of the invention also does not require the use of any device, such as a photodiode or photomultiplier to read the results, thus meeting yet another need for fecal occult blood testing.
[0021] Figure 1 shows a schematic for the method of the current invention for the detection of occult blood. In general, after performing negative and positive control tests, a specimen is deposited, usually through direct defecation, into a suitable receptacle, such as a commode bowl, a bedpan or other receptacle. There the specimen is tested in situ without the need for a tester to remove any portion of the specimen from said receptacle or to physically modify the specimen. Test reagents are added sequentially to the receptacle containing the specimen, the lights extinguished, and the presence or absence of chemiluminescence is noted. Presence of any chemiluminescence in the receptacle with the specimen is indicative of occult blood.
[0022] The schematic for Figure 1 relates to the use of the method for the present invention with a toilet (commode) bowl as the preferred receptacle for the specimen, but it is understood that other receptacles can be used as stated above.
Testing Procedure - Negative control
[0023] Now referring to FIG. 1 . - Testing Procedure Step 1 - Negative Control.
Prior to the introduction of any potentially positive substance, such as stool, urine, or other bodily fluid, a negative control test should be performed in the following manner. In 1A, a sufficient quantity of an oxidizing solution as taught herein is poured into the water of a commode (toilet) bowl. Said quantity of oxidizing solution is of an adequate amount for the approximately 1800 ml_ of water contained in a typical toilet bowl. In 1 B, a predetermined quantity of a chemiluminescent reagent, as taught herein, is poured into the commode. As per 1 C, the room light is turned out, and the toilet bowl is observed.
[0024] Any neon-like glow observed from the commode water with this procedure indicates residual toilet bowl cleaners or trace metals from the plumbing in the commode water. If a blue glowing light occurs with the use of only the oxidizing solution and the chemiluminescent solution (1 E), the patient is instructed to stop the test, and then is further instructed in 1 F to avoid using toilet bowl cleaners, to remove any cleaning products that may be present in the commode tank, and to flush the commode twice a day over a three day period, after which time, the negative control test should be repeated. If chemiluminescence is again present, the cause is the presence of excess trace metals in the water supply and the test for occult blood should not be performed in this commode. To continue the test, the patient must defecate in a bedpan or other receptacle to test for fecal occult blood. Approximately one liter of deionized water (enough to cover the fecal specimen) should be poured over the fecal specimen in the receptacle. Next, the reagents are added per Step 3 of FIG. 1 as if the test were being performed in a toilet bowl. If an alternate receptacle is utilized, the negative and positive control steps should be omitted.
[0025] If no neon-like blue glowing light is seen, the control is considered to be acceptably negative (1 D), and the patient is instructed to flush the toilet twice to remove the reagents from the toilet bowl before proceeding to Testing Procedure Step 2 - Positive Control.
[0026] Any neon-like blue glowing light that may occur when flushing the commode in the dark is to be ignored. Even though a negative control test can be completely negative, a brilliant, blue glowing light may be produced when the commode is flushed. This is due to trace metals from the toilet bowl tank mixing with the reagents and does not represent occult blood in the fecal specimen.
[0027] Testing Procedure - Positive control
[0028] Still referring to FIG. 1 - Testing Procedure Step 2 - Positive Control. If the negative control test is satisfactorily negative, a positive control test is preferably performed to illustrate to the user how a positive test should appear. The positive control may be oxidizing compounds like hypochlorite, iodine, or iodophors. Dried beef blood functions well as a positive control, as does purified human hemoglobin that has been tested free of HIV and hepatitis. Hemin or Sodium and potassium salts of Hemin (Frontier Scientific, Inc. in, Logan, Utah, USA) are the positive controls of choice.
[0029] Per FIG.1 , after the toilet has been flushed twice, an adequate quantity of an oxidizing solution (2A) is added to the commode water. In 2B, a positive control agent (for example, one drop of a Hemin solution from Porphyrin Products, Inc. containing 150 mg hemin/mL dissolved in ethanol) is added to the toilet bowl water. The chemiluminescent or chemifluorescent compound of 2C is sprayed or poured into the toilet bowl water and the room is darkened (2D) prior to reading the test. The chemiluminescence or chemifluorescence detected with the naked eye in the darkened room is noted by the user. When a neon-like glowing blue light is seen in the water and on the surface of the toilet water (2E), the control is considered to be acceptably positive. Now the patient knows what to expect if the fecal specimen contains occult blood. The patient is instructed to flush the toilet twice to remove the reagents from the toilet bowl before proceeding to Testing Procedure Step 3 - Patient Sample. If no neon-like blue glow is seen (2F), the patient is instructed in 2G to stop the test as this indicates inactive reagents, which would render the test invalid. A new kit must be obtained before proceeding.
Testing Procedure - Patient sample
[0030] Still referring to FIG.1 - Testing Procedure Step 3 - Patient Sample. In a preferred method of performing the test for a patient sample, after flushing the toilet twice as per instruction at the end of the positive control step, the patient defecates or
micturates (3A) into the toilet bowl. Next, per 3B, an adequate quantity of the oxidizing solution is added to the toilet bowl water. Lastly, an adequate volume of
chemiluminescent or chemifluorescent compound (3C), preferably sodium luminol, is sprayed or poured into the toilet bowl water and the room is darkened so there is no ambient light(3D). If the fecal or urine sample contains blood or its degradation products like heme, hemoglobin, protoporphyrin IX, or iron, there is an immediate dramatic production of a neon-like blue light visible to the naked eye in the darkened room, which may be seen without the use of photodiodes or photomultipliers. The amount of light production depends on the amount of heme, hemoglobin,
protoporphyrin IX, or iron in the toilet bowl water. If any amount of neon-like glowing blue light is seen, the test is positive (3F) for occult blood and the patient is instructed to contact a physician regarding follow-up testing, such as a colonoscopy. (3G). If the sample in the toilet bowl water does not contain heme, hemoglobin, protoporphyrin IX, or iron, there will be no light visible and the test is considered negative for occult blood (3E).
[0031] The preferred chemiluminescent and/or chemi-fluorescent compound is sodium luminol. In a preferred method, this invention requires the application of an oxidizing compound either just prior or subsequent to micturition or defecation, followed by application of a chemiluminescent or chemifluorescent compound. Alternately, the oxidizing compound and chemiluminescent or chemifluorescent components may be mixed together just prior to application to the urine and/or fecal specimen. The reaction may also be achieved by simply pouring the chemicals into the device containing the specimen if there is enough water in the device..
[0032] One mode of applying the required chemicals is with a bottle fitted with a spray nozzle. The volume delivered by the spray nozzle should be such that approximately five to ten actuations of the spray nozzle are needed to apply an adequate quantity of each chemical for each test. A preferred method is to simply pour an adequate volume of hydrogen peroxide containing a chelator into the container holding the specimen followed by pouring an adequate volume of the chemiluminescent reagent.
[0033] The test for occult blood of the current invention may also be used on aspirates of gastric juice obtained via naso-gastric tubes. The hydrogen peroxide and sodium luminol are poured into the receptacle containing the specimen, the light is turned off and any chemiluminescence is easily determined.
[0034] The test for occult blood of the current invention may also be used on sputum samples. A patient would take an inhalation treatment with hypertonic saline (three percent sodium chloride) administered via a regular nebulizer or ultrasonic nebulizer. The hypertonic saline would induce water into the bronchial tubes and induce sputum production. The sputum is saved in a sterile cup. When an adequate sputum sample is obtained the hydrogen peroxide and sodium Luminol are poured into the cup. The light is turned off. The production of chemiluminescence indicates occult blood in the sputum. The patient should be referred for bronchoscopy. This may lead to an earlier detection of bronchial carcinoma.
[0035] The test for occult blood of the current invention may also be used on urine samples. The urine could be tested in the commode water as one would test for occult blood on fecal samples. Alternatively, a one to two ounce urine specimen could have the oxidizing agent and Sodium Luminol added to the sterile urine cup. The light is turned off. The production of chemiluminescent indicates occult blood in the urine. The patient will require an intravenous pyelogram and perhaps cystoscopy.
[0036] The volume of the reagents necessary for the reaction optimization will vary depending on the volume of the receptacle holding the specimen. The preferred range of volume of the oxidizing reagent for a standard commode bowl is 10-40 ml_ of the 5 % hydrogen peroxide - chelator solution, with 30 ml_ being most preferred. The preferred range of volume of the chemiluminescent reagent for a standard commode bowl is 10- 50 ml_ of the reagent containing 2 mg/mL purified luminol in 75 mg/mL sodium
hydroxide, with 30 ml_ being most preferred.
[0037] If the fecal (stool) specimen contains peroxidases from plants, or catalases then there may be very minuscule light output from a multitude sites on the stool surface. Blood produces a dramatic neon-like blue light output which is sustained and easily seen in a dark room, whereas peroxidases from other sources produce only minuscule, if any, light output. The light output from heme, hemoglobin, protoporphyrin IX, or iron is both dramatic and prolonged. The light output may last as long as ten minutes. The light output may also be reproduced by a second application of the chemiluminescent reagent disclosed herein.
[0038] The method of the invention overcomes a deficiency in other tests for fecal occult blood, i.e., guaiac, which are accompanied by an unacceptably large number of false positives due to dietary peroxidases which are common in onion, bell peppers and
radishes, for example. Human hemoglobin is a pseudo-peroxidase. The false- positives caused by dietary peroxidases are because the ideal pH for performing guaiac tests and detecting dietary sources of peroxidases overlap.
[0039] It has now been found that luminol reacts best with heme when the pH is between 12.00 and 13.00. Dietary peroxidases are inactivated above pH 9.6.
Therefore, in the method of the invention, where testing to detect fecal occult blood takes place at an extremely high pH, a dramatic reduction of the likelihood that a false positive will occur from dietary peroxidases or catalases has been achieved. This will increase specificity over previously reported methods.
Oxidizing Reagent
[0040] In step 3B of FIG. 1 an oxidizing reagent is added to the receptacle holding the specimen. An oxidizing agent is used to lyse any red blood cells present in the specimen in order to release the hemoglobin. Also, the oxidizing compound serves as a catalyst for luminol. The oxidizing reagent is selected from inorganic peroxides, organic peroxides, and mixtures thereof. The oxidizing reagent must further comprise a chelator to bind metal ions which may be present in the toilet water either before or after deposition of the specimen. The same oxidizing reagent is also used in the negative and positive control tests in steps 1A and 2A.
[0041] Examples of inorganic peroxides which may be used are hydrogen peroxide, sodium peroxycarbonate, and sodium perborate or mixtures thereof. Preferred inorganic peroxides are hydrogen peroxide, sodium peroxycarbonate and sodium perborate. The preferred concentration is between three and five percent. Three percent hydrogen peroxide is most preferred.
[0042] The choice of peroxides may influence the choice of chelator utilized as is explained further below. Organic peroxides which may be used include diacyl peroxides, ketone peroxides, peroxydicarbonates, peroxyesters, dialkyl peroxides, hydroperoxides or peroxyketals. Examples of preferred organic peroxides are cumene hydroperoxide, benzoyl peroxide or t-butyl hydroperoxide.
[0043] The oxidizing compound preferably is kept in a separate container from the cyclic hydrazide as mixing the solutions may reduce the shelf life of the hydrazide. The
oxidizing compound may be organic peroxides, inorganic peroxides, or mixtures of the two.
[0044] The peroxide compound may be added as a dry solid, for example,
peroxycarbonate granules, but the preferred embodiment is a liquid containing a trace metal ion chelator. This liquid may be either poured or sprayed into the receptacle for receiving the human excrement. The inorganic peroxide, organic peroxide or mixture of the two, may be added to the water in the toilet bowl either before or after defecation or urination. If the oxidizer is added before the specimen, then following defecation or urination, additional peroxide compound may be applied to the surface of the water. A preferred means of application is for the reagent to be poured into the commode water.
[0045] Due to limited solubility in water, some organic peroxides would float on top of the toilet bowl water. In one embodiment of the invention, such organic peroxides could be placed into the toilet bowl water prior to defecation or urination, and the urine or fecal specimen would pass through the peroxide component as the specimen goes into the toilet bowl water. By passing through the peroxide component, any red blood cells on the surface of the specimen would immediately be lysed. This would be particularly useful when the fecal specimen sinks to the bottom of the toilet bowl. Additional peroxide may be added to the toilet bowl water following defecation or urination to complete the lysis of the red blood cells.
[0046] The simplest oxidizing solution that performs well in the novel assay of this invention is hydrogen peroxide. Over-the-counter three percent (3%) hydrogen peroxide functions well as an oxidizer.
[0047] A trace metal chelator must be added to prevent false-positive reactions in the toilet water. Hydrogen peroxide must not be used with EDTA in the context of a luminol assay for the detection of blood. However, sodium peroxycarbonate and sodium perborate can be used in conjunction with EDTA.
[0048] An acceptable chelator should have a pH in the range of 4 -7, preferably near neutral, and be compatible with the oxidizer component of the oxidizing reagent. To select an appropriate chelator, a simple test, such as follows, may be utilized. The proposed chelator should be mixed with the oxidizer of choice and the mixture capped. After three days, the oxidizer levels are determined using any suitable method for detection of the oxidizer. For example, when hydrogen peroxide is used as the
oxidizer, levels are determined using any suitable method for the determination of hydrogen peroxide. Such methods may include, inter alia, titration, spectrophotometry, fluorescence, chemiluminescence, electrochemical or any other method suitable for detection of hydrogen peroxide. There are also commercial kits available for hydrogen peroxide detection which may be employed. If no substantial change has occurred in hydrogen peroxide levels, the chelator may be used in the method of the present invention.
[0049] One example of a commercially available chelator compatible with hydrogen peroxide is a phosphoric acid-based chelator sold under the tradename DEQUEST FS 0520 as a solution with excellent transition metals chelation properties. (Solutia, Inc., St. Louis, Missouri). This product has a pH of 5.0 and is capable of binding the trace metals in water. The preferred concentration of Dequest FS 0520 is one hundred cc per liter of 3% hydrogen peroxide.
Chemiluminescent Reagent
[0050] In Step 3C of FIG.1 , a reagent comprising a chemiluminescent agent is added to the receptacle containing the specimen. The chemiluminescent agent is selected from the purified sodium or potassium salts of luminol (5-amino-2,3-dihydro 1 ,4- phthalazinedione; o-amino-phthalyhydrazide) and/or the sodium or potassium salts of benzo (ghi) perylene-1 ,2-dicarboxylic acid. Use of the purified luminol reagent as disclosed herein is necessary in the method of the invention in order that the
chemiluminescence produced by the occult blood may be detected by the naked eye in the dark without the aid of instrumentation. The same chemiluminescent reagent is also used in the negative and positive controls in steps 1 B and 2C.
[0051] While sodium luminol is the preferred agent, chemiluminescence may also be achieved with cyclic or acyclic hydrazides. In cyclic hydrazides, analogs with
substituents on the nonheterocyclic ring are easily prepared, but any substitution of the heterocyclic ring renders the compounds non-chemiluminescent. Cyclic hydrazide compounds which are up to 150% more efficient in chemiluminescence than luminol, such as benzo (ghi) perylene-1 ,2-dicarboxylic acid hydrazide, can be prepared according to the method of Wei and White. Tetrahdron Letters: Volume 39: 3559 (1971 ) by C. C. Wei and E. H. White, "An efficient chemiluminescent hydrazide: benzo (ghi) perylene 1 ,2 - dicarboxylic acid hydrazide." Monoacylhyrazides are also reported to be chemiluminescent. Journal of Organic Chemistry Volume 27 (4): 1 198 -1202, 1967 by White, et al. The most efficient monoacylhydrazides prepared are hydrazides of dehydroluciferin and acridine-9-carboxylic acid. These compounds are only about one third as efficient in light production as luminol. Purified sodium luminol or benzo (ghi) perylene 1 ,2 - dicarboxylic acid hydrazide are the preferred compounds for the novel kit.
[0052] Non-interfering substances which can augment the chemiluminescence are contemplated, but the sensitivity should be sufficient as to not require augmenting the chemiluminescence of the luminol.. An example of a non-interfering substance would be fluorscein.
Chemiluminescent Reagent - Chemistry of Luminol
[0053] Chemiluminescence is the emission of light (424 nm wave length) from chemical reactions at ordinary temperatures. One example of chemiluminescence is the oxidation of luminol (5-amino-2,3-dihydro 1 ,4-phthalazinedione; o-amino- phthalylhydrazide), to produce an intense neon-like blue light. Luminol exhibits an intense neon-like blue chemiluminescence by oxidation in an alkaline solution.
[0054] Luminol reacts with animal blood as well as with human blood. The advantage of luminol for the detection of blood is its extreme sensitivity. Hematin can be detected in a dilution of 1 :1 ,000,000 or 10"4 micrograms of crystalline hemoglobin. The duration of the luminescence depends upon the amount of blood present.
[0055] Currently, the primary use of luminol is in forensic science for the detection of blood at crime scenes. Analytical chemists have also used luminol for the detection of trace amounts of iron, cobalt, copper and chromium (III). Free radical generation from
erythrocytes or whole blood has been monitored continuously by luminol-amplified chemiluminescence.
[0056] The reaction path shown in FIG. 2 is proposed as an explanation for the chemiluminescence of luminol at an alkaline pH.
[0057] In the chemiluminescence of luminol in water, a good match has been found for the wavelength distribution of the chemiluminescence, the fluorescence of the total reaction product and the chemiluminescence of sodium aminophthalate. All three emissions are found at 424 nm in water. Furthermore, in mixtures of water and dimethylsulfoxide, or other protic solvents, a double emission occurs in both
chemiluminescence and fluorescence, with peak positions unchanged from the values in the pure solvents (i.e., 485 and 424 nm).
Chemiluminescent Reagent - Purification of Luminol
[0058] After exposure to the purified luminol reagent of the invention, the ambient light is eliminated, and the specimen may be observed with the unaided eye for the presence or absence of a neon-like chemiluminescent blue light. The
chemiluminescence production of a blue light is indicative of blood. The light emission produced with the purified luminol reagent according to the method of the invention is as much as one hundred fold greater than the light emission of unpurified luminol. The reaction of one drop of blood in a flask containing 1800 cc of water demonstrates that purified Luminol can detect 5 nanograms of hemoglobin per ml_. The reaction product can be easily seen with normal vision without the aid of photodiodes or photomultipliers
[0059] An appropriate purity of luminol salt must be utilized. If the luminol starting material has contaminants, then a procedure for purifying luminol must be used. A procedure for the purification of luminol is provided herein. Other purification methods may also be effective. The level of purity can be tested by determining if a known sample comprising a drop of blood oxidized with 3 ml_ of hydrogen peroxide can be detected as stated above with addition of 5 ml_ of an aqueous solution of sodium luminol (2 mg/mL sodium luminol; 75 mg/mL sodium hydroxide) to said oxidized blood with the chemiluminescence visible to the unaided eye.
[0060] When luminol is purchased from a chemical supplier, the bottle usually states that the product is >98% pure. Within that range of purity, however, a variety of crystal
colors may be found. Crystals may appear yellow, orange, greenish-black or cream. Those crystals which are yellow, orange or greenish-black may produce some glow, but the intensity is not as great or as prolonged as that seen with cream to white colored crystals. Luminol that has, orange or greenish-black colored crystals must be purified prior to use in the method of the invention. If the luminol product has a starting purity of >99% with crystals that are cream to white in color, it may be used in the method of invention without further purification providing the chemiluminescence produced is at least 75% of the glow intensity seen with the purified luminol. Luminol crystals with >99% purity demonstrating acceptable chemiluminescence as described herein may be acquired from Gold Biotechnology of St. Louis, MO.
[0061] If the luminol does not perform to the standard indicated above, it may be purified by the following method. In the purification method of the invention crude luminol is first converted to sodium luminol. Crude luminol is dissolved to near saturation in 5% weight/volume room temperature sodium hydroxide (i.e. 100 mg/mL), suction filtered through a 0.22 micron membrane (Millipore), and then crystallized at 0°C for four hours. The sodium luminol precipitates out leaving a supernatant that is solid black. The precipitated crystals are filtered on a Whatman GFA disk, then dissolved in the minimum volume of 5% sodium hydroxide and re-crystallized at 0°C for 18 hours. These crystals are filtered, washed with ice-cold sodium hydroxide, and then dried in the dark. The resulting sodium luminol crystals are dissolved in water (about 100 mg/mL), then one drop of concentrated hydrochloric acid (37%) is added per 10 mL of solution, and the solution is mixed in the dark for thirty minutes. The precipitated base is centrifuged and dried in the dark. The purified sodium luminol appears as white crystals compared to the yellow, orange, or green-black colored crystals of unpurified luminol.
[0062] An alternative method of purifying luminol is as follows. One gram of crude luminol is added to 50 mL of deionized water. Seven mL of 1 Molar sodium hydroxide is added to the solution, followed by the drop-wise addition of 1 mL of concentrated hydrochloric acid (37%). The hydrochloric acid brings the pH back to 7.00. During the addition of hydrochloric acid a white precipitate appears. This is purified luminol. The supernatant is removed by aspiration and the precipitate washed with deionized water. The white precipitate is warmed in an oven to evaporate any remaining water.
Chemiluminescent Reagent - Preparation of Preferred Luminol Reagent
[0063] A chemiluminescent reagent comprising a basic solution of purified sodium luminol is prepared by solubilizing purified sodium luminol in 75 mg/mL of sodium hydroxide to make a solution with a concentration of from about 0.01 to 5 mg/mL sodium luminol. The preferred concentration of the chemiluminescent reagent is about 2.0 mg/mL sodium luminol since above this concentration there is only a slight increase in light output. The near zenith of chemiluminescence of purified sodium luminol occurs with a concentration of 2 mg/mL. The concentration of sodium hydroxide used in the luminol solution should be sufficient to raise the water in the testing system to a pH of greater than 10, and greater or lesser than 75 mg/mL may be required to achieve that pH. For the volume of water in a standard toilet bowl, the most preferred concentration of sodium hydroxide is 75 mg/mL, which is adequate to raise the pH of the water to the optimum pH of 12.4. FIG. 3 shows chemiluminescence vs. time of several luminol concentrations at specified pH values.
[0064] To make one liter of the preferred luminol reagent per Table I below, 75.0 g sodium hydroxide is dissolved in approximately 900 cc of the deoxygenated and deionized water. Then, 2.0 g of the purified sodium luminol is added to the sodium hydroxide solution and stirred. The purified sodium luminol dissolves immediately. The solution is then Q.S. to one liter with deoxygenated and deionized water. Preferably, the mixing is done in a room with very dim or no light as luminol is light sensitive.
Alternatively, prior to the final Q.S. with deionized water, the solution can be Q.S. to pH 12.4 with concentrated hydrochloric acid, per Table II below.
[0065] The average volume of water in a toilet bowl is about 1800 mL. Addition of one ounce (30 mL) of the chemiluminescent reagent containing 2 mg/mL sodium luminol in 75 mg/mL of sodium hydroxide will raise the pH of the toilet water to about 12.4. This is necessary to achieve optimal light output allowing the chemiluminescence to be seen with the naked eye in a darkened room without the need of instrumentation.
[0066] The optimal method for detecting occult blood is to perform the test with a pH greater than 10.0, and preferably at a pH of near 12. 4. At this pH, most, if not all, plant peroxidases will be inactivated allowing maximal chemiluminescence to occur and elimination of the false positives caused by plant peroxidases seen with previous FOBT
performed at pH 9.0 or less. By raising the pH to greater than 12, hydrogen peroxide is converted to perhydroxyl ions. This is the preferred species of hydrogen peroxide for the oxidation of luminol. The pH requirement to reduce or eliminate false-positives due to dietary peroxidases, i.e. > 10 and preferably at pH 12.4, is critical with sodium luminol, which point has not been recognized prior to this invention.
[0067] Tables I and II below provide preferred ingredient ratios for the sodium luminol reagent.
Table I
Table II
Chemiluminescent Reagent - Preparation of Alternative Reagent - Disodium benzyl (ghi) perylene hvdrazide 1 ,2 dicarboxylic acid
[0068] An alternative preferred chemiluminescent reagent is disodium benzyl (ghi) perylene hydrazide 1 ,2 dicarboxylic acid. Formulations for the alternative reagent of the present invention are given in Tables III and IV below.
Table III
[0069] Four grams sodium hydroxide and 8.0 g sodium carbonate are dissolved in approximately 900 cc of water. Then, 1.0 g sodium benzyl (ghi) perylene hydrazide 1 ,2 dicarboxylic acid is added and mixed. Lastly, deionized water is added to make the solution to one liter by volume.
Table IV
[0070] Twelve grams of sodium hydroxide is dissolved in approximately 900 cc of water. Then, 1 .0 g sodium benzyl (ghi) perylene hydrazide 1 ,2 dicarboxylic acid hydrazide is added and mixed. Hydrochloric acid (37%) is used to lower the pH to 12.5. Deionized water is added to make the solution one liter by volume.
[0071] Examples of performance are presented in the in vitro tests given below.
EXAMPLE 1 : OPTIMUM CONCENTRATION OF LUMINOL
[0072] Four solutions of sodium luminol in pH 12.4 sodium hydroxide were prepared with concentrations equaling 0.5 mg/mL, 1 .0 mg/mL, 2.0 mg/mL and 5.0 mg/mL. A few drops of human blood were added to four separate 100 cc Erlenmeyer flasks. Three milliliters of 3% hydrogen peroxide were added to each Erlenmeyer flask and stirred to oxidize the red blood cells. The hydrogen peroxide also functions as a catalyst for the Luminol reaction. Five cc of each prepared sodium luminol solution was added to the appropriate Erlenmeyer flask to begin the chemiluminescence. The results were interpreted by intensity of chemiluminescence. A small increase in chemiluminescence was seen when the sodium luminol was increased from 0.5 mg/mL to 1 .0 mg/mL. A doubling of light intensity was observed in the flask with 2.0 mg/mL sodium luminol over that seen in the flask with 1 .0 mg/mL. The maximum light output was achieved with 5.0 mg/mL, but this was only marginally better than was seen with the 2.0 mg/mL sodium luminol solution. Thus, the optimum concentration of sodium luminol for light output was determined to be 2.0 mg/mL. The results are shown in FIG. 3. The light output was read at 424 nm.
EXAMPLE 2: PATIENT WITH MICROSCOPIC HEMATURIA
[0073] A twenty-seven year old white male was diagnosed with a urethral stricture. Under general anesthesia, repeated urethral dilatation was performed with filliforms and followers. For the first three days post-procedure, gross (observable by the naked eye) hematuria occurred. The patient waited an additional three days until the urine appeared to be completely normal. One week following urethral dilatation the patient performed a test of the present invention for the presence of occult blood in the urine. One ounce of a solution of 3% hydrogen peroxide was poured into the commode water. The patient then voided all of his urine into the commode. Lastly, an aqueous solution of sodium luminol (2 mg/mL sodium luminol; 75 mg/mLsodium hydroxide) was sprayed onto the top of the commode water. The bathroom light was turned off. A bright chemiluminescent blue light occurred around the edge of the top of the commode water at the interface of the water and wall of the commode, which indicated the presence of occult blood in the urine. The glowing blue light was easily seen with the naked eye.
EXAMPLE 3: IN VITRO TEST WITH HUMAN BLOOD IN A COMMODE
[0074] A solution of 5% hydrogen peroxide was poured into a two-ounce bottle fitted with a Calmar ® SSA 0.025 spray nozzle (Calamar, Lee's Summit, MO). The hydrogen peroxide solution was prepared by diluting a 50% hydrogen peroxide (Food Grade - Solvay Interox) solution one to ten with deionized water. An aqueous sodium luminol solution (2.0 mg/mL sodium luminol in 2 Molar sodium hydroxide) was also poured into a two ounce bottle fitted with a Calmar ® SSA 0.025 spray nozzle.
[0075] Ten sprays of the hydrogen peroxide were applied to the top of the water in a commode. One drop of human blood was applied via a 21 -gauge needle. Ten sprays of the sodium luminol/sodium hydroxide solution were applied to the top of the water in a commode. With the room light turned out, a brilliant, chemiluminescent blue light glowed around the top edge of the commode water that was easily visible to the naked eye. The reaction was such that a blue, glowing halo was produced. There was also some blue light glowing from the commode water. The glowing blue light lasted for over ten minutes. Once the glowing blue light had faded, a repeat application of ten sprays of sodium luminol/sodium hydroxide solution again produced a blue light, which was equal in intensity to the first reaction.
EXAMPLE 4: PLANT PEROXIDASES
[0076] Turnips, radishes, carrots, apples, horseradish sauce, cantaloupe, watermelon, and celery were purchased from a grocery store. Each vegetable was ground into a slurry with a blender. For each vegetable tested, two ounces of hydrogen peroxide was poured into the commode water, and then the vegetable slurry was added. An aqueous solution of sodium luminol (1 mg/mL sodium luminol; 14 mg/mL sodium hydroxide) was sprayed into the commode water. The bathroom light was then turned off and the water was observed for the blue glow of chemiluminescence. If the number of "30" was arbitrarily assigned to the chemiluminescence produced by one drop of human blood, then by comparison, turnips produced a "2", radishes produced a "1 ", and carrots produced a "0.5". All of the other vegetables were negative for any
chemiluminescence. It is important to note that even when the vegetables produced a blue light, the light was dull and lasted less than half a minute. Human blood control
produced a bright, glowing light that was sustained for up to ten minutes and was easily visible to the naked eye.
EXAMPLE 5: FALSE POSITIVES WITH FLUSHING THE COMMODE
[0077] Commode water will test negative for chemiluminescence when the oxidizing solution containing chelators and then the sodium luminol/sodium hydroxide solution are sprayed on the water's surface unless the water has been contaminated by toilet bowl cleaners. It has been observed that some commodes will produce a brilliant, glowing blue light during drainage of the commode water during flushing. This is thought to occur due to trace metal contamination from the toilet bowl tank.
EXAMPLE 6: FALSE POSITIVES WITH TOILET BOWL CLEANERS
[0078] Commodes that have been disinfected with hypochlorites may produce a false positive reaction. The reaction with hypochlorites is intense, but brief, lasting less than five seconds. Therefore, false positives from toilet bowl cleaners containing
hypochlorites can be determined by the distribution, intensity, and length of reaction.
EXAMPLE 7: INCREASED LIGHT PRODUCTION WITH USE OF LUMINOL
REAGENT ADJUSTED WITH HYDROCHLORIC ACID TO PH 12.5
[0079] Fifteen milliliters of 3% hydrogen peroxide was added to the commode water. One drop of human blood from a 22 gauge needle was added to the commode water. Sodium luminol (2mg/ml_) in 2 Molar sodium hydroxide, with or without the pH adjusted to 12.4 using concentrated hydrochloric acid, was added to the commode water. The light was turned out. The pH 12.4 sodium luminol solution that contained hydrochloric acid glowed approximately five times brighter than the sodium luminol solution without hydrochloric acid.
EXAMPLE 8: DETECTION OF POLYPS IN A 70 YEAR OLD MAN
[0080] A 70-year-old man desiring to check his feces for occult blood in the privacy of his own home performed the chemiluminescence assay of the invention for occult blood. After defecation, he poured one ounce of 3% hydrogen peroxide in the commode water. Next, he added 2 cc of purified sodium luminol solution to the commode water. Lastly, he turned out the light. The edge of the fecal specimen produced a brilliant blue light indicative of occult blood. He was referred to a
gastroenterologist for colonoscopy. At colonoscopy he was found to have three adenomatous polyps in the descending and sigmoid colon which were removed by snare and cautery. The polyps were 1 .5 cm in diameter and all were benign on pathological examination.
EXAMPLE 9: COMPARISON OF SENSITIVITY OF CHEMILUMINESCENCE
VERSUS GUAIAC -BASED TEST IN VITRO
[0081] An aliquot of blood from an EDTA tube was obtained. The hemoglobin (Hgb) was 15.4 g/dL and the hematocrit was 45.3%. Five Styrofoam containers numbered consecutively were prepared. One hundred cc of deionized water was placed in cup number one, while 90 cc were placed in each subsequent cup. Using a mechanical pipette, 20 microliters of blood were added to cup number one and thoroughly mixed. Ten cc from cup number one were subsequently added to cup number two and thoroughly mixed. The same procedure was followed until cup number five, taking 10 cc from the cup just mixed and mixing the aliquot thoroughly with the water in the next cup. Each cup was also labeled with the concentration which was as follows:
Cup 1 3.00 milligrams of Hgb per ml_
Cup 2 0.30 milligrams of Hgb per ml_
Cup 3 0.03 milligrams of Hgb per ml_
Cup 4 3.00 micrograms of Hgb per ml_
Cup 5 0.30 micrograms of Hgb per ml_
[0082] Five commercially available guaiac-based test cards (HEMOCCULT®) were obtained and were also numbered consecutively one through five. One cc of fluid from each cup was used to thoroughly soak the cards. These were allowed to air dry. The developer was then used per the package directions.
[0083] The assay of the novel chemiluminescent invention was performed by adding 3 cc of 3% hydrogen peroxide to each cup and mixing. In a dark room, 0.6 cc of purified
sodium luminol solution was added to each cup. The results are as follows:
Table V
Chemiluminescence vs. Guaiac-Based Testing In Vitro
[0084] Thus, the novel invention is at least 200,000 times more sensitive for the detection of hemoglobin than the commercially available guaiac test kits This is important since adenomatous polyps less than 2.5 cm in diameter only lose small amounts of blood, i.e., 1 to 2 cc per day. The commercially available HEMOCCULT® test (Beckman-Colter) requires 10 cc of blood in a fecal specimen in order to provide correct results about 50% of the time.
[0085] In another experiment, two 2000 mL Erienmeyer flasks were used to simulate a toilet bowl. Eighteen hundred mL of deionized water was added to each flask to duplicate the average amount of water found in a commode. Human hemoglobin was diluted to 5 nanograms/mL in the first flask and 1 nanogram/mL of water in the second flask. One ounce of three percent hydrogen peroxide was added to each flask. One ounce of Luminol was added to each flask. The ambient light was extinguished.
Chemiluminescence was visible when there were only 5 nanograms of hemoglobin per mL but was not visible at 1 nanogram of hemoglobin per mL. Thus, the procedure of the invention can detect hemoglobin at a concentration of 5 nanograms per mL and may be able to detect concentrations in excess of 1 nanograms per mL.
EXAMPLE 10 - DUPLICATE TESTING IN 50 PATIENTS
[0086] Fifty volunteers over the age of fifty compared the novel chemiluminescence assay of the invention to a commercially available immunochemical test kit. The novel
chemiluminescent assay of the present invention was performed as described in Example 8 on three consecutive bowel movements, while a commercially available immunochemistry test kit (InSure® Fecal Immunochemistry Test (FIT) (Enterix, Inc, Edison, N.J.) was employed on the first two fecal specimens. The FIT test was negative for all fifty patients. The assay of the invention was positive for three volunteers. For one patient, all three fecal specimens were positive using the assay of the invention. For the other two patients, two out of three fecal specimens were positive for occult blood using the assay of the invention.
[0087] All fifty patients had total colonoscopies. The forty-seven patients who were negative for both tests also had negative colonoscopies. All three patients who were positive by the chemiluminescence test were discovered to have adenomatous polyps. Two of the patients had the polyps in the sigmoid colon and one patient had a polyp in the cecum. All were removed by polypectomy.
EXAMPLE 11 - DUPLICATE TESTING IN 30 PATIENTS
[0088] The novel FOBT was performed on patients scheduled for elective colonoscopy at the Gl Center in Overland Park, Kansas. Volunteers signed informed consent to perform FOBT with the chemiluminescence test of the present invention as well as a commercially available fecal immunochemistry test kit (FIT) (InSure ® brand, Enterix, Inc, Edison, N.J.). The FDA pre-approved product instructions for experimental use of the chemiluminescence test of the invention, and the patients were provided with these instructions. The patients were required to complete an exit questionnaire to ascertain that they understood the instructions. A diet free of red meat, ascorbic acid, iron supplements and vitamins was recommended for three days prior to any stool testing and through the testing period. Volunteers were instructed follow the procedure for observing the negative control before proceeding with the test samples.
[0089] Volunteers were instructed to use the chemiluminescence test kit on three consecutive bowel movements prior to colonoscopy since colonic bleeding from polyps may occur intermittently.
[0090] Two bowel movements were also tested for FOBT using the commercially- available fecal immunochemistry test (FIT) kit (InSure ®, Enterix, Inc.). Instructions accompanying the commercial kit were followed to obtain appropriate specimens. This entailed a brush collection technique of commode water adjacent a fecal specimen.
The brush was used to transfer the water specimen to a card slide, which was mailed according to the protocol provided to an off-site laboratory for testing. The testing results were obtained and compiled in Table VI below.
[0091] To perform the chemiluminescence test, one 30 cc bottle of chemiluminescence Oxidizing Solution was poured into the commode water containing the stool. Next, one 30 cc bottle of chemiluminescence Developer Solution was poured into the commode water. The bathroom light was turned off. The presence or absence of
chemiluminescence was recorded on a 4 x 6 card and returned to the physicians at the Endoscopy Imaging Center in Overland Park, Kansas upon out-patient visit for colonoscopy. The data was compiled in Table VI below.
[0092] Thirty-four volunteers (20 females and 10 males) age 50 or older, with an average age of 63.2, agreed to participate in the study. Four volunteers did not return testing results to the Endoscopy Imaging Center, leaving thirty sets of results for evaluation.
[0093] The chemiluminescence test kit was positive in eight cases. The commercial fecal immunochemistry test (FIT) kit was negative in all thirty of the volunteers. Results are presented in Tables VI and VII below.
Table VI
(-) = negative Chemiluminescence; (++) = positive Chemiluminescence;
Representing test 1 , test 2, test 3
[0094] Only limited conclusions can be drawn from these data since the sample size of patients is small, however, it is notable that the chemiluminescence assay detected a number of bleeding lesions whereas the immunochemical assay was not positive in any patient. The false-negative results of the InSure® test were probably caused by sampling the wrong portion of feces.
[0095] There was one 66 year old female volunteer who had one positive
chemiluminescent test on the second bowel movement but with negative results on the first and third bowel movements. The colonoscopy only revealed diverticulosis.
Diverticula may bleed, however, no bleeding was observed during colonoscopy. The patient may not have been compliant with the dietary restriction or may have had bleeding in the upper gastrointestinal tract or small bowel. An upper endoscopy was not performed, but probably should be considered when a patient has a positive chemiluminescent test and a negative colonoscopy.
[0096] Two females with positive chemiluminescent tests were found to only have hemorrhoids at colonoscopy. One volunteer had three positive chemiluminescent test results and the other had one out of three tests positive. Bleeding from hemorrhoids may cause positive chemiluminescence tests although this is not indicative of CRC.
[0097] It is a notable finding that in this small study the chemiluminescence test detected several adenomatous polyps. The smallest adenomatous polyp detected was 6 mm. The other four patients' adenomatous polyps were all 10 mm. All adenomatous polyps were removed with a snare and were free of metaplasia or dysplasia.
Interestingly, the chemiluminescence test was positive when polyps were located in the cecum, hepatic flexure, descending colon and rectal area. The chemiluminescence test did not detect any hemorrhage from four patients who had 3 mm sessile polyps. These preliminary data suggest that the chemiluminescence test has the ability to detect premalignant lesions in all locations with greater sensitivity than the immunochemical assay and with a high rate of specificity. Interestingly, the 6 mm adenomatous polyp was positive on all three bowel movements. If the chemiluminescent test can detect adenomatous polyps where bleeding from a 1 cm polyp is estimated to be 1 .2 cc per day then it should routinely detect a mucosal adenocarcinoma where the bleeding is estimated to be about 5 cc per day. With a standard guaiac test, such as Hemoccult®, blood loss of approximately 10 cc per day is needed for the test to correctly detect bleeding in 50% of the samples.
[0098] The use of aspirin or other non-steroidal anti-inflammatory drugs has been shown to cause bleeding in the stomach or gastrointestinal tract of susceptible individuals. Discontinuing aspirin therapy prior to FOBT in those individuals may prevent erroneous readings. It has also been shown that use of a low-dose aspirin regimen can lower colorectal cancer risk. If aspirin therapy leads to bleeding from polyps, continuing aspirin therapy prior to FOBT could enhance detection of polyps. With this information in mind, the physician can make the determination on whether a patient should continue or discontinue aspirin therapy prior to fecal testing with the method of the invention.
Use of Phenalphthalin to detect plant peroxidases in the specimen
[0099] An additional step may be included in the method of the invention utilizing a chromogenic composition which will change colors in the presence of plant
peroxidases. This chromogenic composition would also change colors in the presence of occult blood following the addition of hydrogen peroxide to lyse the red blood cells. The addition of this step would further reduce the incidence of false positives by identifying samples that contain active plant peroxidases, the presence of which could lead to false positive test results.
[00100] The preferred chromogenic composition comprises an aqueous basic solution of phenolphthalin. The sodium salt of phenolphthalin is colorless in deoxygenated
alkaline solution, and is readily oxidized by minute quantities of blood in the presence of hydrogen peroxide to phenolphthalein which gives a deep hot pink color in alkaline solution. If plant peroxidases are present in the fecal specimen, the colorless phenolphthalin will oxidize to phenolphthalein producing a hot pink color which is visible to the unaided eye in ambient room lighting. Following the addition of hydrogen peroxide, occult blood will also produce a bright pink glow. If occult blood or plant peroxidases are not present, the composition will remain clear.
[00101] In this method, after the appropriate negative and positive controls have been satisfactorily completed, the feces is deposited in the toilet bowl receptacle, an alkaline solution containing approximately 2 mg/mL of phenolphthalin in 0.25 moles/L sodium or potassium hydroxide is added to the receptacle containing the specimen, and the receptacle is observed for 30-45 seconds. If a color change to hot pink is visible, plant peroxidases are present in the sample and the test should be discontinued. Appropriate dietary recommendations should be implemented to reduce these peroxidases before retesting. If no hot pink color is seen, approximately one ounce (30 mL) of 3% hydrogen peroxide is added to the receptacle with the specimen and the specimen is observed for 30-45 seconds for a color change. If the hot pink color is visible, the test is positive for occult blood and no further reagents need to be added. If no hot pink color is seen, a luminol solution containing 2 mg/mL luminol in 75 mg/mL sodium hydroxide is added to the receptacle, the light is turned out and the receptacle is observed for the blue glow of the chemiluminescence. If the blue glow is seen, the test is positive for occult blood and the patient should be referred for further testing. If no blue glow is seen, the test is negative for occult blood.
[00102] It is contemplated that the reagents appropriate for a test procedure can be supplied in a kit comprising a container for oxidizing agent, a container of
chemiluminescent reagent, and instructions for carrying out a method of FOBT testing on a fecal specimen deposited in a toilet bowl by a person in the normal manner. The kit preferably further comprises containers of reagent(s) which test for interfering substances in the toilet bowl and/or peroxidases as described above.
Claims
1 . A method for detecting occult blood in bodily excrement from a patient
comprising the steps of: a) adding an oxidizing reagent effective for lysing red blood cells which may be present in said excrement to a zone in which a bodily excrement specimen has been pre-deposited;
b) simultaneously or subsequently adding a chemiluminescent reagent to said zone;
c) reducing or eliminating any ambient light present in the vicinity of said zone; d) observing with the unaided eye for the presence or absence of a
chemiluminescence, which presence is indicative of the presence of occult blood in said specimen and which absence is indicative of the absence of blood in said specimen.
2. The method of claim 1 wherein said zone is a toliet bowl into which said excrement was pre-deposited by said patient.
3. The method of claim 1 wherein said chemiluminescent reagent is selected from the group consisting of purified sodium luminol and purified potassium luminol.
4. The method of claim 1 , wherein said chemiluminescent reagent is selected from the group consisting of cyclic hydrazides, acyclic hydrazides, sodium salts of benzo (ghi)perylene-1 ,2-dicarboxylic acid and potassium salts of benzo (ghi)perylene-1 ,2- dicarboxylic acid.
5. The method of Claim 3, wherein said reagent has a pH greater than 9.0.
6. The method of Claim 1 , wherein said oxidizing reagent is selected from the group consisting of inorganic peroxides, organic peroxides, and mixtures thereof.
7. The method of Claim 6, wherein said oxidizing reagent is an inorganic peroxide selected from the group consisting of hydrogen peroxide, sodium peroxycarbonate, sodium perborate and mixtures thereof.
8. The method of Claim 6, wherein a preferred amount of said oxidizing agent is from about three to about five percent.
9. The method of Claim 7, wherein said oxidizing agent is hydrogen peroxide.
10. The method of Claim 6, wherein said peroxide is an organic peroxide selected from the group consisting of diacyl peroxides, ketone peroxides, peroxydicarbonates, peroxyesters, dialkyl peroxides, hydroperoxides and peroxyketals.
1 1 . The method of Claim 6 wherein said peroxide is an organic peroxides selected from the group consisting of cumene hydroperoxide, benzoyl peroxide and t-butyl hydroperoxide.
12. The method of Claim 1 further comprising a trace metal chelator.
13. The method of Claim 12, wherein said oxidizing agent is selected from sodium peroxycarbonate and sodium perborate and said trace metal chelator comprises EDTA.
14. The method of Claim 12, wherein said oxidizing agent is hydrogen peroxide and said trace metal chelator is devoid of EDTA.
15. The method of Claim 12, wherein said trace metal chelator has a pH in the range of 4 -7 and is compatible with the oxidizer component of the oxidizing reagent.
16. The method of Claim 15 wherein said trace metal chelator pH is near neutral.
17. The method of Claim 1 wherein said oxidizing reagent further comprises a chelator for metal ions, which chelator is compatible with hydrogen peroxide.
18. The method of Claim 17 wherein said chelator is Dequest 0520.
19. The method of Claim 3 wherein the pH in the test zone is at least 12.
20. The method of Claim 3 wherein the pH in the test zone is at least 12.4.
21 . The method of Claim 1 , further comprising the step of testing for plant
peroxidases by employing a pre-step of adding a chromogenic composition comprising an aqueous basic solution of phenolphthalin to said reaction zone in order to determine if the patient's sample is suitable for testing with the chemiluminescent reagent.
22. A kit for use in a method of detecting occult blood in bodily excrement from a patient in a toilet bowl reaction zone in which a specimen of excrement has been pre- deposited, comprising: a) one or more containers of oxidizing reagent, said oxidizing reagent effective for lysing red blood cells which may be present in said excrement to a zone in which a bodily excrement specimen has been pre-deposited;
b) one or more containers of chemiluminescent reagent; and
c) instructions for carrying out the method of Claim 1 .
23. The kit of Claim 22, wherein each container contains a pre-measured amount of reagent appropriate for use in the toilet bowl reaction zone
24. The kit of Claim 23, further comprising containers of control reagents.
25. The kit of Claim 24, further comprising a container of reagent comprising a chromogenic composition which will change colors in the presence of plant
peroxidases.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US32003810P | 2010-04-01 | 2010-04-01 | |
| US61/320,038 | 2010-04-01 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2011123776A2 true WO2011123776A2 (en) | 2011-10-06 |
| WO2011123776A3 WO2011123776A3 (en) | 2012-02-09 |
Family
ID=44712855
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/030934 WO2011123776A2 (en) | 2010-04-01 | 2011-04-01 | Method for detecting occult blood |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011123776A2 (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013082267A1 (en) | 2011-11-30 | 2013-06-06 | Wheeldon Eric B | Apparatus and method for the remote sensing of blood in human feces and urine |
| US20140147924A1 (en) * | 2011-11-30 | 2014-05-29 | Eric B. Wheeldon | Apparatus and Method for the Remote Sensing of Blood in Human Feces and Urine |
| CN105738328A (en) * | 2014-12-10 | 2016-07-06 | 胡朝阳 | A household networked system for detecting occult blood in feces and urine |
| JPWO2017038711A1 (en) * | 2015-08-28 | 2017-08-31 | 栄研化学株式会社 | Reagent composition for immunological measurement and use thereof |
| KR20180107277A (en) * | 2016-02-16 | 2018-10-01 | 메트리오팜 아게 | A new crystalline form of 5-amino-2,3-dihydropthalazine-1,4-dione |
| JP2019504865A (en) * | 2016-02-16 | 2019-02-21 | メトリオファーム アーゲー | Process for producing crystalline form of 5-amino-2,3-dihydrophthalazine-1,4-dione |
| US10575830B2 (en) | 2015-02-25 | 2020-03-03 | Outsense Diagnostics Ltd. | Bodily emission analysis |
| CN112858269A (en) * | 2020-12-25 | 2021-05-28 | 广东绿康医疗器械有限公司 | Preparation method and application method of detection reagent for nasopharyngeal mucosa cell free heme |
| US11467091B2 (en) | 2016-08-30 | 2022-10-11 | Outsense Diagnostics Ltd. | Bodily emission analysis |
| US11971356B2 (en) | 2022-12-21 | 2024-04-30 | Outsense Diagnostics Ltd. | Bodily emission analysis |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4578358A (en) * | 1983-05-03 | 1986-03-25 | Warner-Lambert Company | Collection of specimens and detection of occult blood therein |
| US4725553A (en) * | 1983-09-29 | 1988-02-16 | Helena Laboratories Corporation | Test composition for detecting occult blood |
| US5273888A (en) * | 1984-01-16 | 1993-12-28 | Helena Laboratories Corporation | Chemical test kit and method for determining the presence of blood in a specimen and for verifying the effectiveness of the chemicals |
| US5840584A (en) * | 1995-06-07 | 1998-11-24 | Waldenburg; Ottfried | Blood in feces test device |
| US20070037296A1 (en) * | 2005-08-12 | 2007-02-15 | Leslie Goulden | Combined chemical and immunochemical fecal occult blood test |
-
2011
- 2011-04-01 WO PCT/US2011/030934 patent/WO2011123776A2/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4578358A (en) * | 1983-05-03 | 1986-03-25 | Warner-Lambert Company | Collection of specimens and detection of occult blood therein |
| US4725553A (en) * | 1983-09-29 | 1988-02-16 | Helena Laboratories Corporation | Test composition for detecting occult blood |
| US5273888A (en) * | 1984-01-16 | 1993-12-28 | Helena Laboratories Corporation | Chemical test kit and method for determining the presence of blood in a specimen and for verifying the effectiveness of the chemicals |
| US5840584A (en) * | 1995-06-07 | 1998-11-24 | Waldenburg; Ottfried | Blood in feces test device |
| US20070037296A1 (en) * | 2005-08-12 | 2007-02-15 | Leslie Goulden | Combined chemical and immunochemical fecal occult blood test |
Cited By (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140147924A1 (en) * | 2011-11-30 | 2014-05-29 | Eric B. Wheeldon | Apparatus and Method for the Remote Sensing of Blood in Human Feces and Urine |
| US8802442B2 (en) * | 2011-11-30 | 2014-08-12 | Eric B. Wheeldon | Apparatus and method for the remote sensing of blood in human feces and urine |
| CN104254621A (en) * | 2011-11-30 | 2014-12-31 | 埃里克·B·惠尔顿 | Apparatus and method for the remote sensing of blood in human feces and urine |
| EP2785877A4 (en) * | 2011-11-30 | 2015-06-17 | Eric B Wheeldon | Apparatus and method for the remote sensing of blood in human feces and urine |
| AU2012345944B2 (en) * | 2011-11-30 | 2018-11-01 | Robert J. Barsotti | Apparatus and method for the remote sensing of blood in human feces and urine |
| WO2013082267A1 (en) | 2011-11-30 | 2013-06-06 | Wheeldon Eric B | Apparatus and method for the remote sensing of blood in human feces and urine |
| CN105738328A (en) * | 2014-12-10 | 2016-07-06 | 胡朝阳 | A household networked system for detecting occult blood in feces and urine |
| US10575830B2 (en) | 2015-02-25 | 2020-03-03 | Outsense Diagnostics Ltd. | Bodily emission analysis |
| US11786224B2 (en) | 2015-02-25 | 2023-10-17 | Outsense Diagnostics Ltd. | Bodily emission analysis |
| US11129599B2 (en) | 2015-02-25 | 2021-09-28 | Outsense Diagnostics Ltd. | Bodily emission analysis |
| JPWO2017038711A1 (en) * | 2015-08-28 | 2017-08-31 | 栄研化学株式会社 | Reagent composition for immunological measurement and use thereof |
| CN107923907A (en) * | 2015-08-28 | 2018-04-17 | 荣研化学株式会社 | Immunologic measure reagent composition and application thereof |
| JP2019504865A (en) * | 2016-02-16 | 2019-02-21 | メトリオファーム アーゲー | Process for producing crystalline form of 5-amino-2,3-dihydrophthalazine-1,4-dione |
| JP2019509268A (en) * | 2016-02-16 | 2019-04-04 | メトリオファーム アーゲー | Process for producing crystalline form of 5-amino-2,3-dihydrophthalazine-1,4-dione |
| JP2022037005A (en) * | 2016-02-16 | 2022-03-08 | メトリオファーム アーゲー | Crystal form of 5-amino-2,3-dihydrophthalazine-1,4-dione |
| JP7034079B2 (en) | 2016-02-16 | 2022-03-11 | メトリオファーム アーゲー | Method for producing crystalline form of 5-amino-2,3-dihydrophthalazine-1,4-dione |
| KR102535960B1 (en) * | 2016-02-16 | 2023-05-23 | 메트리오팜 아게 | New crystalline form of 5-amino-2,3-dihydrophthalazine-1,4-dione |
| JP7331067B2 (en) | 2016-02-16 | 2023-08-22 | メトリオファーム アーゲー | Crystal form of 5-amino-2,3-dihydrophthalazine-1,4-dione |
| KR20180107277A (en) * | 2016-02-16 | 2018-10-01 | 메트리오팜 아게 | A new crystalline form of 5-amino-2,3-dihydropthalazine-1,4-dione |
| US11467091B2 (en) | 2016-08-30 | 2022-10-11 | Outsense Diagnostics Ltd. | Bodily emission analysis |
| US11561181B2 (en) | 2016-08-30 | 2023-01-24 | Outsense Diagnostics Ltd. | Bodily emission analysis |
| CN112858269A (en) * | 2020-12-25 | 2021-05-28 | 广东绿康医疗器械有限公司 | Preparation method and application method of detection reagent for nasopharyngeal mucosa cell free heme |
| US11971356B2 (en) | 2022-12-21 | 2024-04-30 | Outsense Diagnostics Ltd. | Bodily emission analysis |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2011123776A3 (en) | 2012-02-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2011123776A2 (en) | Method for detecting occult blood | |
| EP0121317B2 (en) | Peroxidase activity detection composition | |
| Gray et al. | Atypical mycobacterial infection of the gastrointestinal tract in AIDS patients. | |
| Rockey et al. | Detection of upper gastrointestinal blood with fecal occult blood tests | |
| Gordon | Malignant neoplasms of the colon | |
| EP2259714B1 (en) | Device for detecting a medical condition or disease | |
| JPH09505405A (en) | Screening test for early detection of colorectal neoplasms | |
| JP2007535665A (en) | Apparatus and method for detecting the presence of hemoglobin in a biological sample | |
| US4675160A (en) | Occult blood test monitor | |
| Harewood et al. | Detection of occult upper gastrointestinal tract bleeding: performance differences in fecal occult blood tests | |
| Jekarl et al. | Evaluation of a newly developed rapid stool antigen test using an immunochromatographic assay to detect Helicobacter pylori | |
| US4719181A (en) | Free flowing granular indicator material for peroxidase-like activity | |
| AU646525B2 (en) | Peroxidase indicator system for basic media | |
| US20120135529A1 (en) | Detection of Occult Blood in Feces or Urine | |
| Hardcastle et al. | Screening for colorectal cancer: a critical review | |
| Sanford et al. | Fecal occult blood testing | |
| JP2665249B2 (en) | Method for detecting carboxylic acid in a sample | |
| Ostrow | Tests for fecal occult blood | |
| Jaffe et al. | A new occult blood test not subject to false-negative results from reducing substances | |
| Cooper et al. | Evaluation of the guaiac fecal occult blood test for detection of gastrointestinal bleeding in the rhesus macaque (Macaca mulatta) | |
| EP3896448A1 (en) | A method for determining likelihood of an inflammatory gastrointestinal tract disease | |
| Dybdahl et al. | Screening for occult faecal blood loss in a community by means of Hemoccult® II slides and a tetramethylbenzidine test | |
| Häkkinen et al. | Screening of colorectal tumours using an improved faecal occult blood test. Quantitative aspects. | |
| Madadi | Development of 2-D and 3-D Paper-based Microfluidic devices for the detection of Cryptosporidium and Giardia | |
| Mettlin et al. | The current status of early detection and screening for colorectal cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11763508 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11763508 Country of ref document: EP Kind code of ref document: A2 |