NITROSATED AND NITROSYLATED STEROIDS FOR THE TREATMENT OF CARDIOVASCULAR DISEASES AND DISORDERS
FIELD OF THE INVENTION
The present invention relates to nitrosated and /or nitrosylated steroids and to methods for the treatment of cardiovascular diseases and disorders, particularly the prophylactic and /or therapeutic treatment of restenosis, by administering nitrosated and /or nitrosylated steroids that are capable of releasing nitric oxide or indirectly delivering or transferring nitric oxide to targeted sites under physiological conditions. The methods for the treatment of cardiovascular diseases and disorders may further comprise administering at least one compound that donates, transfers, or releases nitric oxide and /or elevate endogenous nitric oxide or endothelium- derived relaxing factor in vivo and/or is a substrate for nitric oxide synthase.
BACKGROUND OF THE INVENTION Endothelium-derived relaxing factor (EDRF) is a vascular relaxing factor secreted by the endothelium and is important in the control of vascular tone, blood pressure, inhibition of platelet aggregation, inhibition of platelet adhesion, inhibition of mitogenesis, inhibition of proliferation of cultured vascular smooth muscle, inhibition of leukocyte adherence and prevention of thrombosis. EDRF has been identified as nitric oxide (NO) or a closely related derivative thereof (Palmer et al, Nature, 327:524-526 (1987); Ignarro et al, Proc. Natl. Acad. Sci. USA, 84:9265-9269 (1987)). Removal of the endothelium is a potent stimulus for neointimal proliferation, a common mechanism underlying the restenosis of atherosclerotic vessels after balloon angioplasty (Liu et al., Circulation, 79:1374-1387 (1989); Ferns et al., Science, 253:1129-1132 (1991)). Balloon arterial injury results in endothelial denudation and subsequent regrowth of dysfunctional endothelium (Saville, Analyst, 83:670-672 (1958)) that may contribute to the local smooth muscle cell proliferation and extracellular matrix production that result in reocclusion of the arterial lumen. Nitric oxide dilates blood vessels (Vallance et al, Lancet, 2:997-1000 (1989)) inhibits platelet activation and adhesion (Radomski et al., Br. J Pharmacol, 92:181-187 (1987))
and nitric oxide limits the proliferation of vascular smooth muscle cells in vitro (Garg et al., /. Clin. Invest., 83:1774-1777 (1986)). Similarly, in animal models, suppression of platelet-derived mitogens decreases intimal proliferation (Ferns et al., Science, 253:1129-1132 (1991)). The potential importance of endothelium-derived nitric oxide in the control of arterial remodeling after injury is further supported by recent preliminary reports in humans suggesting that systemic NO donors reduce angiographic restenosis six months after balloon angioplasty (The ACCORD Study Investigators, /. Am. Coil. Cardiol. 23:59 . (Abstr.) (1994)).
Another aspect of restenosis may simply be mechanical, e.g., caused by the elastic rebound of the arterial wall and /or by dissections in the vessel wall caused by the angioplasty procedure. These mechanical problems have been successfully addressed by the use of stents to tack-up dissections and prevent elastic rebound of the vessel thereby reducing the level of re-occlusion for many patients. The stent is typically inserted by catheter into a vascular lumen and expanded into contact with the diseased portion of the arterial wall, thereby providing internal support for the lumen. No material has, however, been developed that matches the blood- compatible surface of the endothelium. In fact, in the presence of blood and plasma proteins, artificial surfaces are an ideal setting for platelet deposition (Salzman et al, Phil. Trans. R. Soc. Lond., B294:389-398 (1981)). Exposure of blood to an artificial surface initiates reactions that lead to clotting or platelet adhesion and aggregation. Within seconds of blood contact, the artificial surface becomes coated with a layer of plasma proteins which serves as a new surface to which platelets readily adhere, become activated, and greatly accelerate thrombus formation (Forbes et al, Brit. Med. Bull, 34(2):201-207 (1978)). Despite considerable efforts to develop nonthrombogenic materials, no synthetic material has been created that is free from this effect. In addition, the use of anticoagulant and platelet inhibition agents has been less than satisfactory in preventing adverse consequences resulting from the interaction between blood and artificial surfaces. Consequently, a significant need exists for the development of additional methods for preventing platelet deposition and thrombus formation on artificial surfaces.
Apparatuses and methods have been developed for delivering nitric oxide- releasing compounds and other drugs selectively and locally to a specific internal
body site, e.g., for preventing restenosis after percutaneous transluminal coronary angioplasty. U.S. Patent Nos. 5,428,070, 5,861,168 and 5,945,452 describe the treatment of vascular degenerative diseases by the use of orally or intramurally administered L-arginine as a dietary supplement to enhance nitric oxide production by providing the substrate of nitric oxide synthase, the enzyme which metabolizes L- arginine to L-citrulline and nitric oxide. This treatment is inappropriate for restenosis, as the endothelium cell levels of L-arginine are not diminished, but the specific isoform of nitric oxide synthase localized in the endothelium cells is dysfunctional. In addition replacement therapy using dietary L-arginine is an inappropriate treatment since cellular sources of L-arginine arise primarily from the reverse metabolism of L-citrulline to L-arginine (Sessa et al, Proc. Natl. Acad Sci. USA, 87:8607-8611 (1990)). U.S. Patent Nos. 5,282,785 and 5,286,254 describe drug delivery apparatuses for delivering drugs in a radially restricted manner and across selectively permeable membranes, respectively. The apparatuses have several disadvantages, as do most intra vascular devices, by promoting platelet deposition at the site the device is located or after removal of a device at a vascular site.
A number of compounds have been developed which are capable of delivering nitric oxide, including compounds which release nitric oxide upon being metabolized and compounds which release nitric oxide spontaneously in aqueous solutions. However, the pharmacological applications of these compounds have been limited by their tendency to distribute evenly throughout the medium. Another limitation of these nitric oxide compounds is their propensity to rapidly release the nitric oxide, thereby necessitating frequent dosing to achieve a prolonged biological effect. Thus, there is a need for nitric-oxide releasing compounds which are capable of targeting the nitric oxide released to a particular site of application.
There is also a need in the art for effective methods of preventing and treating cardiovascular diseases and disorders. The present invention is directed to these, as well as other, important ends.
SUMMARY OF THE INVENTION The present invention describes novel nitrosated and /or nitrosylated steroids and methods for preventing and /or treating cardiovascular diseases and disorders by administering one or more nitrosated and /or nitrosylated steroids that are capable of releasing a therapeutically effective amount of nitric oxide to a targeted
site effected by a cardiovascular disease or disorder. Preferably, the methods of the present invention are used for treating and /or preventing restenosis.
Another embodiment of the present invention describes methods for preventing and /or treating cardiovascular diseases and disorders by administering one or more nitrosated and /or nitrosylated steroids that are capable of releasing a therapeutically effective amount of nitric oxide to a targeted site effected by a cardiovascular disease or disorder in combination with at least one compound that donates, transfers, or releases nitric oxide and/or stimulates the endogenous production of nitric oxide or EDRF in vivo and /or is a substrate for nitric oxide synthase.
Another embodiment of the present invention describes compositions and methods for making compositions comprising nitrosated and /or nitrosylated steroids that are bound to a natural or synthetic matrix, which can be applied with specificity to a biological site of interest. For example, the matrix containing the nitrosated and /or nitrosylated steroid can be used to coat the surface of a medical device or instrument that comes into contact with blood (including blood components, blood products and the like) or vascular tissue.
Yet another embodiment of the present invention describes methods for the prevention of platelet aggregation and platelet adhesion caused by the exposure of blood to a medical device or instrument by incorporating nitrosated and /or nitrosylated steroids that are capable of releasing a therapeutically effective amount of nitric oxide into and /or on the portion(s) of the medical device that come into contact with blood (including blood components and blood products) or vascular tissue. Another aspect of the invention relates to the local administration of nitrosated and/or nitrosylated steroids to treat injured tissue, such as damaged blood vessels.
These and other aspects of the present invention are explained in detail below. BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a dose response curve of human coronary artery smooth muscle cells for the compound of Example 1 (i.e., 9 -fluoro-16α-methyl-llβ,17 ,21-trihydroxy- pregna-l,4-diene-3,20-dione-21-nitrate ester) and the parent steroid (i.e.,
dexamethasone) .
Fig. 2 is a dose response curve of human coronary artery smooth muscle cells for the compound of Example 2 (i.e., llβ,17α,21-trihydroxy-l,4-pregnadiene-3,20- dione-21 -nitrate ester) and the parent steroid (i.e., prednisolone). DETAILED DESCRIPTION OF THE INVENTION
As used throughout the disclosure, the following terms, unless otherwise indicated, shall be understood to have the following meanings.
"Cardiovascular disease or disorder" refers to any cardiovascular disease or disorder known in the art, including, for example, restenosis, atherogenesis, angina, (particularly chronic, stable angina pectoris), ischemic disease, congestive heart failure or pulmonary edema associated with acute myocardial infarction, atherosclerosis, thrombosis, controlling blood pressure in hypertension (especially hypertension associated with cardiovascular surgical procedures), platelet aggregation, platelet adhesion, smooth muscle cell proliferation, vascular complications associated with the use of medical devices, wounds associated with the use of medical devices, and the like. Complications associated with the use of medical devices may occur as a result of increased platelet deposition, activation, thrombus formation or consumption of platelets and coagulation proteins. Such complications, which are within the definition of "cardiovascular disease or disorder," include, for example, myocardial infarction, pulmonary thromboembolism, cerebral thromboembolism, thrombophlebitis, thrombocytopenia, bleeding disorders and/or any other complications which occur either directly or indirectly as a result of the foregoing disorders.
"Restenosis" is a cardiovascular disease or disorder that refers to the closure of a peripheral or coronary artery following trauma to the artery caused by an injury such as, for example, angioplasty, balloon dilation, atherectomy, laser ablation treatment or stent insertion. For these angioplasty procedures, restenosis occurs at a rate of about 30-60% depending upon the vessel location, lesion length and a number of other variables. Restenosis can also occur following a number of invasive surgical techniques, such as, for example, transplant surgery, vein grafting, and the like.
"Artificial surface" refers to any synthetic material contained in a device or apparatus that is in contact with blood, vasculature or other tissues. "Blood"
includes blood products, blood components and the like.
"Platelet adhesion" refers to the contact of a platelet with a foreign surface, including any artificial surface, such as a medical device or instrument, as well as an injured vascular surfaces, such as collagen. Platelet adhesion does not require platelet activation. Unactivated, circulating platelets will adhere to injured vascular surfaces or artificial surfaces via binding interactions between circulating von Willdebrand factor and platelet surface glycoprotein Ib/IX.
"Platelet aggregation" refers to the binding of one or more platelets to each other. Platelet aggregation is commonly referred to in the context of generalized atherosclerosis, not with respect to platelet adhesion on vasculature damaged as a result of physical injury during a medical procedure. Platelet aggregation requires platelet activation which depends on the interaction between the ligand and its specific platelet surface receptor.
"Passivation" refers to the coating of a surface which renders the surface non-reactive.
"Platelet activation" refers either to the change in conformation (shape) of a cell, expression of cell surface proteins (e.g., the lib /Ilia receptor complex, loss of GPIb surface protein), and secretion of platelet derived factors (e.g., serotonin, growth factors). "Patient" refers to animals, preferably mammals, more preferably humans.
"Medical device" refers to intravascular or extravascular medical devices, medical instruments, foreign bodies and the like. Examples of intravascular medical devices and instruments include balloons or catheter tips adapted for insertion, prosthetic heart valves, sutures, synthetic vessel grafts, stents (e.g. Palmaz-Schatz stent), arteriovenous shunts, artificial heart valves, foreign bodies introduced surgically into the blood vessels or at vascular sites, leads, pacemakers, implantable pulse generators, implantable cardiac defibrillators, pacemaker cardioverter defibrillators, defibrillators, spinal stimulators, brain stimulators, sacral nerve stimulators, chemical sensors, and the like. Examples of extravascular medical devices and instruments include plastic tubing, dialysis bags or membranes whose surfaces come in contact with the blood stream of a patient.
"Prodrug" refers to a compound that is made more active in vivo.
"Nitric oxide adduct" or "NO adduct" refers to compounds and functional
groups which, under physiological conditions, can donate, release and /or directly or indirectly transfer any of the three redox forms of nitrogen monoxide (NO+, NO", NO*), such that the biological activity of the nitrogen monoxide species is expressed at the intended site of action. "Nitric oxide releasing" or "nitric oxide donating" refers to methods of donating, releasing and/or directly or indirectly transferring any of the three redox forms of nitrogen monoxide (NO+, NO-, NO»), such that the biological activity of the nitrogen monoxide species is expressed at the intended site of action.
"Nitric oxide donor" or "NO donor" refers to compounds that donate, release and /or directly or indirectly transfer a nitrogen monoxide species, and /or stimulate the endogenous production of nitric oxide or endothelium-derived relaxing factor (EDRF) in vivo and/or elevate endogenous levels of nitric oxide or EDRF in vivo. "NO donor" also includes compounds that are substrates for nitric oxide synthase.
"Parent steroid" refers to a steroid that does not have a nitric oxide adduct linked (either directly or indirectly) thereto.
"Alkyl" refers to a lower alkyl group, a haloalkyl group, a lower alkenyl group, a lower alkynyl group, a bridged cycloalkyl group, a cycloalkyl group or a heterocyclic ring, as defined herein.
"Lower alkyl," alone or in combination, refers to branched or straight chain acyclic alkyl groups comprising 1 to 10 carbon atoms, preferably 1 to about 8 carbon atoms, more preferably 1 to about 6 carbon atoms. Exemplary lower alkyl groups include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl, pentyl, iso-amyl, hexyl, octyl, neopentyl and the like.
"Lower alkenyl" refers to a branched or straight chain C2-C10 hydrocarbon (preferably a C2-C8 hydrocarbon, more preferably a C2 to hydrocarbon) which can comprise one or more carbon-carbon double bonds. Exemplary lower alkenyl groups include propylenyl, buten-1-yl, isobutenyl, penten-1-yl, 2,2-methylbuten-l- yl, 3-methylbuten-l-yl, hexan-1-yl, hepten-1-yl and octen-1-yl and the like.
"Lower alkynyl" refers to an unsaturated acyclic C2-C10 hydrocarbon group (preferably a C2-C8 hydrocarbon, more preferably a C2 to C6 hydrocarbon) which can comprise one or more carbon-carbon triple bonds. Exemplary lower alkynyl groups include ethynyl, propynyl, butyn-1-yl, butyn-2-yl, pentyn-1-yl, pentyn-2-yl, 3- methylbutyn-1-yl, hexyn-1-yl, hexyl-2-yl, hexyn-3-yl, 3,3-dimethyl-butyn-l-yl and
the like.
"Alkoxy" alone or in combination, refers to R50O-, wherein R50 is an alkyl group, as defined herein. Exemplary alkoxy groups include methoxy, ethoxy, t-butoxy, cyclopentyloxy and the like. "Acyloxy" refers to an alkanoyl group with 2 to about 5 carbon atoms.
Exemplary acyloxy groups include acetyloxy, propanoyloxy, butanoyloxy, benzoyloxy and the like.
"Alkylsilyloxy" refers to an alkylsilyl group wherein the alkyl is an defined above, preferably having 3 to 8 carbon atoms. Exemplary alkylsilyloxy groups include trimethylsilyl, t-butyldimethylsilyl and the like.
"Haloalkyl" refers to a lower alkyl group, an lower alkenyl group, an lower alkynyl group, a bridged cycloalkyl group, a cycloalkyl group or a heterocyclic ring, as defined herein, to which is appended one or more halogens, as defined herein. Exemplary haloalkyl groups include trifluoromethyl, chloromethyl, 2-bromobutyl, 1- bromo-2-chloro-pentyl and the like.
"Lower thioalkyl" refers to an alkoxyl group wherein sulfur replaces oxygen. "Alicyclic hydrocarbon" refers to an aliphatic group in a ring with 3 to about 10 carbon atoms, preferably from 3 to about 6 carbon atoms. Exemplary alicyclic hydrocarbon groups include cyclopropyl, cyclopropylenyl, cyclobutyl, cyclopentyl, cyclohexyl, 2-cyclohexen-l-ylenyl, cyclohexenyl and the like.
"Bridged cycloalkyl" refers to two or more cycloalkyl groups, heterocyclic groups, or a combination thereof fused via adjacent or non-adjacent atoms. Bridged cycloalkyl groups can be unsubstituted or substituted with one, two or three substituents independently selected from alkyl, alkoxy, amino, alkylamino, dialkylamino, hydroxy, halo, carboxyl, alkylcarboxylic acid, aryl, amidyl, ester, alkylcarboxylic ester, carboxamido, alkylcarboxamido, oxo and nitro. Exemplary bridged cycloalkyl groups include adamantyl, decahydronapthyl, quinuclidyl, 2,6- dioxabicyclo[3.3.0]octane, 7-oxabycyclo[2.2.1]heptyl, 8-azabicyclo[3,2,l]oct-2-enyl and the like. "Cycloalkyl" refers to a saturated or unsaturated cyclic hydrocarbon comprising from about 3 to about 8 carbon atoms. Cycloalkyl groups can be unsubstituted or substituted with one, two or three substituents independently selected from alkyl, alkoxy, amino, alkylamino, dialkylamino, arylamino,
diarylamino, alkylarylamino, aryl, amidyl, ester, hydroxy, halo, carboxyl, alkylcarboxylic acid, alkylcarboxylic ester, carboxamido, alkylcarboxamido, oxo and nitro. Exemplary cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl, cyclohepta,l,3-dienyl, and the like. "Heterocyclic ring or group" refers to a saturated, unsaturated cyclic or polycyclic hydrocarbon group having about 3 to about 12 carbon atoms (preferably about 4 to about 6 carbon atoms) where 1 to about 4 carbon atoms are replaced by one or more nitrogen, oxygen and/or sulfur atoms. Sulfur maybe in the thio, sulfinyl or sulfonyl oxidation state. The heterocyclic ring or group can be fused to an aromatic hydrocarbon group. Heterocyclic groups can be unsubstituted or substituted with one, two or three substituents independently selected from alkyl, alkoxy, amino, alkylamino, dialkylamino, arylamino, diarylamino, alkylarylamino, hydroxy, oxo, thial, halo, carboxyl, carboxylic ester, alkylcarboxylic acid, alkylcarboxylic ester, aryl, arylcarboxylic acid, arylcarboxylic ester, amidyl, ester, carboxamido, alkylcarboxamido, arylcarboxamido, sulfonic acid, sulfonic ester, sulfonamido and nitro. Exemplary heterocyclic groups include pyrrolyl, 3- pyrrolinyl,4,5,6-trihydro-2H-pyranyl, pyridinyl, 1,4-dihydropyridinyl, pyrazolyl, triazolyl, pyrimidinyl, pyridazinyl, oxazolyl, thiazolyl, imidazolyl, indolyl, thiophenyl, furanyl, tetrhydrofuranyl, tetrazolyl, 2-pyrrolinyl, 3-pyrrolinyl, pyrrolindinyl, oxazolindinyl 1,3-dioxolanyl, 2,6-dioxabicyclo[3,3,0]octanyl, 2- imidazonlinyl, imidazolindinyl, 2-pyrazolinyl, pyrazolidinyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, 1,3,4-thiadiazolyl, 2H-pyranyl, 4H- pyranyl, piperidinyl, 1,4-dioxanyl, morpholinyl, 1,4-dithianyl, thiomorpholinyl, pyrazinyl, piperazinyl, 1,3,5-triazinyl, 1,3,5-trithianyl, benzo(b)thiophenyl, benzimidazolyl, quinolinyl, and the like.
"Heterocyclic compounds" refer to mono- and polycyclic compounds comprising at least one aryl or heterocyclic ring.
"Aryl" refers to a monocyclic, bicyclic, carbocyclic or heterocyclic ring system comprising one or two aromatic rings. Exemplary aryl groups include phenyl, pyridyl, napthyl, quinoyl, tetrahydronaphthyl, furanyl, indanyl, indenyl, indoyl, and the like. Aryl groups (including bicylic aryl groups) can be unsubstituted or substituted with one, two or three substituents independently selected from alkyl, alkoxy, amino, alkylamino, dialkylamino, arylamino, diarylamino, alkylarylamino,
hydroxy, carboxyl, carboxylic ester, alkylcarboxylic acid, alkylcarboxylic ester, aryl, arylcarboxylic acid, arylcarboxylic ester, alkylcarbonyl, arylcarbonyl, amidyl, ester, carboxamido, alkylcarboxamido, carbomyl, sulfonic acid, sulfonic ester, sulfonamido and nitro. Exemplary substituted aryl groups include tetrafluorophenyl, pentafluorophenyl, sulfonamide, alkylsulfonyl, arylsulfonyl, and the like.
"Alkylaryl" refers to an alkyl group, as defined herein, to which is appended an aryl group, as defined herein. Exemplary alkylaryl groups include benzyl, phenylethyl, hydroxybenzyl, fluorobenzyl, fluorophenylethyl, and the like.
"Arylalkyl" refers to an aryl radical, as defined herein, attached to an alkyl radical, as defined herein.
"Cycloalkylalkyl" refers to a cycloalkyl radical, as defined herein, attached to an alkyl radical, as defined herein.
"Heterocyclicalkyl" refers to a heterocyclic ring radical, as defined herein, attached to an alkyl radical, as defined herein. "Arylheterocyclic ring" refers to a bi- or tricyclic ring comprised of an aryl ring, as defined herein, appended via two adjacent carbon atoms of the aryl ring to a heterocyclic ring, as defined herein. Exemplary arylheterocyclic rings include dihydroindole, 1, 2,3,4- tetra-hydroquinoline, and the like.
"Alkoxy" refers to R50O-, wherein R50 is an alkyl group, as defined herein. Exemplary alkoxy groups include methoxy, ethoxy, t-butoxy, cyclopentyloxy, and the like.
"Arylalkoxy or alkoxyaryl" refers to an alkoxy group, as defined herein, to which is appended an aryl group, as defined herein. Exemplary arylalkoxy groups include benzyloxy, phenylethoxy, chlorophenylethoxy, and the like. "Alkoxyalkyl" refers to an alkoxy group, as defined herein, appended to an alkyl group, as defined herein. Exemplary alkoxyalkyl groups include methoxymethyl, methoxyethyl, isopropoxymethyl, and the like.
"Alkoxy haloalkyl" refers to an alkoxy group, as defined herein, appended to a haloalkyl group, as defined herein. Exemplary alkoxyhaloalkyl groups include 4- methoxy-2-chlorobutyl and the like.
"Cycloalkoxy" refers to R540-, wherein R54 is a cycloalkyl group or a bridged cycloalkyl group, as defined herein. Exemplary cycloalkoxy groups include cyclopropyloxy, cyclopentyloxy, cyclohexyloxy, and the like.
"Haloalkoxy" refers to a haloalkyl group, as defined herein, to which is appended an alkoxy group, as defined herein. Exemplary haloalkyl groups include 1,1,1-trichloroethoxy, 2-bromobutoxy, and the like.
"Hydroxy" refers to -OH. "Oxo " refers to =0.
"Oxy " refers to -O R77 wherein R77 is an organic or inorganic cation.
"Organic cation" refers to a positively charged organic ion. Exemplary organic cations include alkyl substituted ammonium cations, and the like.
"Inorganic cation" refers to a positively charged metal ion. Exemplary inorganic cations include Group I metal cations such as for example, sodium, potassium, and the like.
"Hydroxyalkyl" refers to a hydroxy group, as defined herein, appended to an alkyl group, as defined herein.
"Amino" refers to -NH2. "Nitrate" refers to -0-N02.
"Nitrite" refers to -O-NO.
"Thionitrate" refers to -S-N02.
"Thionitrite" and "nitrosothiol" refer to -S-NO.
"Nitro" refers to the group -N02 and "nitrosated" refers to compounds that have been substituted therewith.
"Nitroso" refers to the group -NO and "nitrosylated" refers to compounds that have been substituted therewith.
"Nitrile" and "cyano" refer to -CN.
"Halogen" or "halo" refers to iodine (I), bromine (Br), chlorine (Cl), and/or fluorine (F).
"Alkylamino" refers to R50NH-, wherein R50 is an alkyl group, as defined herein. Exemplary alkylamino groups include methylamino, ethylamino, butylamino, cyclohexylamino, and the like.
"Arylamino" refers to R55NH-, wherein R55 is an aryl group, as defined herein. "Dialkylamino" refers to R50R52N-, wherein R50 and R52 are each independently an alkyl group, as defined herein. Exemplary dialkylamino groups include dimeth lamino, diethylamino, methyl propargylamino, and the like.
"Diarylamino" refers to Rs^oN-, wherein R55 and R60 are each independently
an aryl group, as defined herein.
"Alkylarylamino" refers to R^R^N-, wherein R^ is an alkyl group, as defined herein, and R55 is an aryl group, as defined herein.
"Aminoalkyl " refers to an amino group, an alkylamino group, a dialkylamino group, an arylamino group, a diarylamino group, an alkylarylamino group or a heterocyclic ring, as defined herein, to which is appended an alkyl group, as defined herein.
"Aminoaryl " refers to an amino group, an alkylamino group, a dialkylamino group, an arylamino group, a diarylamino group, an alkylarylamino group or a heterocyclic ring, as defined herein, to which is appended an aryl group, as defined herein.
"Thio" refers to -S-.
"Sulfinyl" refers to -S(O)-.
"Methanthial" refers to -C(S)-. "Thiai" refers to =S.
"Sulfonyl" refers to -S(0)2\
"Sulfonic acid" refers to -S(0)2OR76, wherein R76 is a hydrogen, an organic cation or an inorganic cation.
"Alkylsulfonic acid" refers to a sulfonic acid group, as defined herein, appended to an alkyl group, as defined herein.
"Arylsulfonic acid" refers to an sulfonic acid group, as defined herein, appended to an aryl group, as defined herein
"Sulfonic ester" refers to -S(0)2OR58, wherein R58 is an alkyl group, an aryl group, an alkylaryl group or an aryl heterocyclic ring, as defined herein. "Sulfonamido" refers to -S(0)2-N(R51)(R57), wherein R51 and R57 are each independently a hydrogen atom, an alkyl group, an aryl group, an alkylaryl group, or an arylheterocyclic ring, as defined herein, and R51 and R57 when taken together are a heterocyclic ring, a cycloalkyl group or a bridged cycloalkyl group, as defined herein. "Alkylsulfonamido" refers to a sulfonamido group, as defined herein, appended to an alkyl group, as defined herein.
"Arylsulfonamido" refers to a sulfonamido group, as defined herein, appended to an aryl group, as defined herein.
"Alkylthio" refers to R50S-, wherein R50 is an alkyl group, as defined herein.
"Arylthio" refers to R55S-, wherein R^ is an aryl group, as defined herein.
"Alkylsulfinyl" refers to R50-S(O)-, wherein R50 is an alkyl group, as defined herein. "Alkylsulfonyl" refers to R50-S(O)2-, wherein R50 is an alkyl group, as defined herein.
"Arylsulfinyl" refers to R55-S(0)-, wherein R55 is an aryl group, as defined herein.
"Arylsulfonyl" refers to R55-S(0)2-, wherein R55 is an aryl group, as defined herein.
"Amidyl" refers to R51C(0)N(R57)- wherein R51 and R57 are each independently a hydrogen atom, an alkyl group, an aryl group, an alkylaryl group, or an arylheterocyclic ring, as defined herein.
"Ester" refers to R51C(0)0- wherein R5] is a hydrogen atom, an alkyl group, an aryl group, an alkylaryl group, or an arylheterocyclic ring, as defined herein.
"Carbamoyl" refers to -0-C(0)N(R51)(R57), wherein R51 and R57 are each independently a hydrogen atom, an alkyl group, an aryl group, an alkylaryl group or an arylheterocyclic ring, as defined herein, or R51 and R57 taken together are a heterocyclic ring, a cycloalkyl group or a bridged cycloalkyl group, as defined herein.
"Carboxyl" refers to -C(0)OR76, wherein R76 is a hydrogen, an organic cation or an inorganic cation, as defined herein.
"Carbonyl" refers to -C(O)-.
"Alkylcarbonyl" refers to R50-C(O)-, wherein R50 is an alkyl group, as defined herein.
"Arylcarbonyl" refers to R55-C(0)-, wherein R55 is an aryl group, as defined herein.
"Carboxylic ester" refers to -C(0)OR58, wherein R58 is an alkyl group, an aryl group, an alkylaryl group or an aryl heterocyclic ring, as defined herein. "Alkylcarboxylic acid" and "alkylcarboxyl" refer to an alkyl group, as defined herein, appended to a carboxyl group, as defined herein.
"Alkylcarboxylic ester" refers to an alkyl group, as defined herein, appended to a carboxylic ester group, as defined herein.
"Arylcarboxylic acid" refers to an aryl group, as defined herein, appended to a carboxyl group, as defined herein.
"Arylcarboxylic ester" and "arylcarboxyl" refer to an aryl group, as defined herein, appended to a carboxylic ester group, as defined herein. . "Carboxamido" refers to -C(0)N(R51)(R57), wherein R51 and R57 are each independently a hydrogen atom, an alkyl group, an aryl group, an alkylaryl group or an arylheterocyclic ring, as defined herein, and R51 and R57 when taken together are a heterocyclic ring, a cycloalkyl group or a bridged cycloalkyl group, as defined herein. "Alkylcarboxamido" refers to an alkyl group, as defined herein, appended to a carboxamido group, as defined herein.
"Arylcarboxamido" refers to an aryl group, as defined herein, appended to a carboxamido group, as defined herein.
"Urea" refers to -N(R59)-C(0)N(R51)(R57) wherein R^, R57, and R59 are each independently a hydrogen atom, an alkyl group, an aryl group, an alkylaryl group, or an arylheterocyclic ring, as defined herein, or R51 and R57 taken together are a heterocyclic ring, a cycloalkyl group or a bridged cycloalkyl group, as defined herein.
Compounds of the present invention that have one or more asymmetric carbon atoms may exist as the optically pure enantiomers, pure diastereomers, mixtures of enantiomers, mixtures of diastereomers, racemic mixtures of enantiomers, diastereomeric racemates or mixtures of diastereomeric racemates. It is to be understood that the present invention anticipates and includes within its scope all such isomers and mixtures thereof. The present invention is based on the discovery that it is possible to treat and /or prevent cardiovascular diseases and disorders in patients by administering one or more steroids that are linked (directly or indirectly) to one or more nitric oxide adducts. Preferably, the steroids that are linked to one or more nitric oxide adducts are administered in the form of a pharmaceutical composition that further comprises a pharmaceutically acceptable carrier or diluent.
Compounds that donate, transfer or release nitric oxide species in vivo have a wide spectrum of advantages and applications. The present invention is also based on the discovery that it is possible to administer at least one steroid, that is
optionally linked to one or more nitric oxide adducts, and at least one nitric oxide donor to prevent and /or treat cardiovascular diseases and disorders, such as, for example, restenosis.
In one embodiment, cardiovascular diseases or disorders may be prevented or treated with compositions comprising (i) one or more steroids that are linked to one or more nitric oxide adducts, and, optionally, (ii) one or more nitric oxide donors. In another embodiment, cardiovascular diseases or disorders may be prevented or treated by separately (including simultaneously) administering (i) one or more steroids that are optionally linked to one or more nitric oxide adducts, and (ii) one or more nitric oxide donors. In either embodiment, the compounds and /or compositions may be in the form of a kit, which can include other components. Such other components in the kit can include, for example, other compounds (including the therapeutic compounds described herein), compositions, a device(s) for administering the compounds and /or compositions, and written instructions for use.
The compositions of the present invention can be used to treat numerous cardiovascular diseases or disorders including, for example, restenosis, atherogenesis, angina (particularly chronic, stable angina pectoris), ischemic disease, congestive heart failure or pulmonary edema associated with acute myocardial infarction, atherosclerosis, thrombosis, controlling blood pressure in hypertension (especially hypertension associated with cardiovascular surgical procedures), platelet aggregation, platelet adhesion, smooth muscle cell proliferation, serious vascular complications associated with the use of medical devices and wounds associated with the use of medical devices. Serious vascular complications can occur as a result of increased platelet deposition, activation, thrombus formation or consumption of platelets and coagulation proteins. Such serious vascular complications, which are well known to one of ordinary skill in the art, include, for example, myocardial infarction, pulmonary thromboembolism, cerebral thromboembolism, thrombophlebitis, thrombocytopenia, bleeding disorders and any additional complication which occurs either directly or indirectly as a result of the foregoing diseases or disorders.
The steroids used in the present invention are preferably hydroxyl-containing steroidal hormones. Hydroxyl-containing steroidal hormones are known in the art
and are described, for example, in the Merck Index on CD-ROM, Twelfth Edition, Version 12:1, (1996), and U.S. Patent No. 5,837,698, the disclosures of which are incorporated by reference herein in their entirety.
Hydroxyl-containing steroidal hormones include, for example, 21- acetoxypregnenolone, alcolometasone, algestone, amcinonide, beclomethasone, betamethasone, budesonide, chlorprednisone, clobetasol, clobentasone, clocortolone, cloprednol, corticosterone, cortisine, corticazol (cortivatol), deflazacort, desonide, desoximetasone, dexamethasone, diflorasone, diflucortolone, difluprednate, enoxolone, fluzacort, flucloronide, flumethasone, flunisolide, flucinolone acetonide, fluocininide, fluocortin butyl, fluocortolone, fluorometholone, fluperolone acetate, fluprednidene acetate, fluprednisolone, flurandrenolide, fluticasone propionate, fluticasone propionate, formocortal, halcinonide, halobetasol propionate, halometasone, haloprednone acetate, hydrocortamate, hydrocortisone and its derivatives (such as phosphate, 21-sodium succinate and the like), hydrocortisone terbutate, isoflupredone, loteprednol etabonate, mazip redone, medrysone, meprednisone, methylprednisolone, mometasone furoate, paremethasone, prednicarbate, prednisolone and its derivatives (such as 21-stearoylglycolate, sodium phosphate and the like), prednisone, prednival, prednylidene and its derivatives (such as 21-diethylaminoactetate and the like), rimexolone, tixocortol, trimcinolone and its derivatives (such as acetonide, benetonide and the like), and the like. Preferred examples of hydroxyl-containing steroidal hormones are glucocorticoids and synthetic steroidal compounds with glucocorticoid activity.
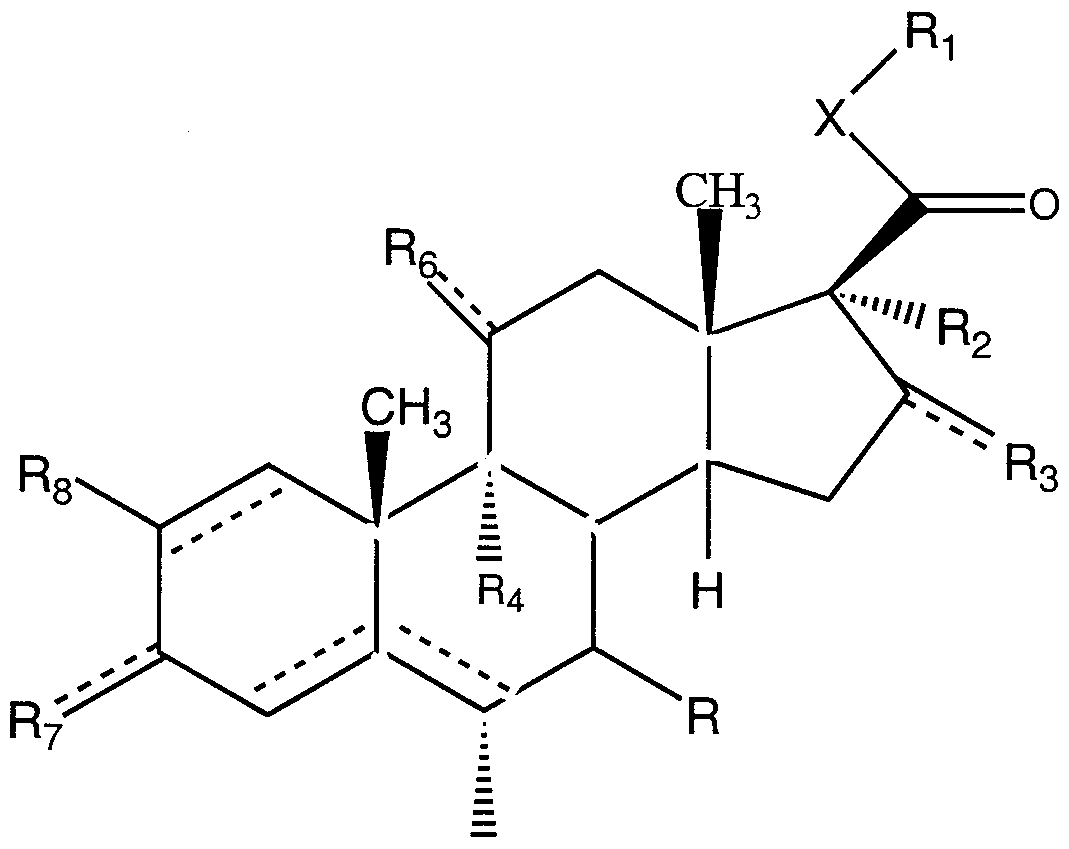
In one embodiment, the present invention describes nitrosated and /or nitrosylated steroids and pharmaceutically acceptable salts thereof of formula (I):
R,
(I)
wherein the dotted lines indicate a single or double bond; R is a hydrogen or halogen;
R, is a hydrogen, a hydroxy, a nitrite ester, a nitrate ester, a thionitrite ester, a thionitrate ester, a halogen, a haloalkyl group, a heterocyclic group (preferably of 2 to 5 carbon atoms, more preferably 3 to 4 carbon atoms, and preferably having 1 or 2 hetero atoms), a nitroxylalkanoyl group (preferably of 2 to about 6 carbon atoms, more preferably 2 to 4 carbon atoms), a sulfhydryl group, a lower thioalkyl group (preferably of 1 to about 6 carbon atoms), an alkoxy group (preferably of 1 to 6 carbon atoms, more preferably 1 to 4 carbon atoms), an alkylsilyloxy group (preferably of 3 to 8 carbon atoms, more preferably 3 to 6 carbon atoms), a lower alkyl group (preferably 1 to 6 carbon atoms, more preferably 1 to 4 carbon atoms), a lower alkenyl group, a lower alkynyl group, wherein each of these group is independently and optionally substituted with a hydroxy group, a halogen (preferably a chloro or fluoro group), a lower alkyl group, a lower alkenyl group, a lower alkynyl group, an alkoxy group, an amino group, a nitro group, a nitrile group, a carboxyl group, a haloalkyl group, or -OC(0)-Rg, wherein R9 is an alkanoic acid group (preferably of 2 to about 6 carbon atoms, more preferably of 2 to 4 carbon atoms), a lower alkyl group (preferably of 1 to about 6 carbon atoms, more preferably 1 to 4 carbon atoms), a lower alkenyl group (preferably of 2 to about 6
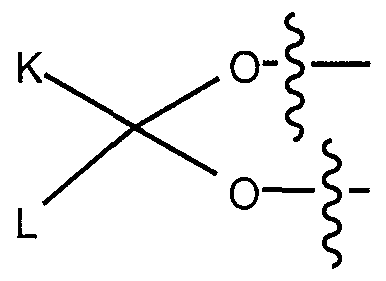
carbon atoms, more preferably of 2 to 4 carbon atoms), a lower alkynyl group (preferably of 2 to about 6 carbon atoms, more preferably of 2 to 4 carbon atoms) or an alkoxy group (preferably of 1 to about 6 carbon atoms, more preferably of 1 to 4 carbon atoms); R2 and R3 are each independently a hydrogen, a hydroxy group, a nitrite ester, a nitrate ester, a nitroxylalkanoyl group (preferably of 2 to about 6 carbon atoms, more preferably of 2 to 4 carbon atoms), a lower alkyl group (preferably of 1 to about 6 carbon atoms, more preferably 1 to 4 carbon atoms), a lower alkenyl group (preferably of 2 to about 6 carbon atoms, more preferably of 2 to 4 carbon atoms), a lower alkynyl group (preferably of 2 to about 6 carbon atoms, more preferably of 2 to 4 carbon atoms), an alkoxy group (preferably of 1 to about 6 carbon atoms, more preferably of 1 to 4 carbon atoms), wherein each of these groups is independently and optionally substituted with a hydroxy group, a lower alkyl group, a lower alkenyl group, a lower alkynyl group, an alkoxy group, an amino group, a nitro group, a nitrile group, a carboxy group and a haloalkyl group, or a group of formula -OC(O)-R10 or -SC(O)-R10, wherein R10 is 2-furanyl, a lower alkyl group (preferably of 1 to about 6 carbon atoms, more preferably of 1 to 4 carbon atoms), or an alkoxy group (preferably of 1 to about 6 carbon atoms, more preferably of 1 to 4 carbon atoms); R2 and R3 may optionally form a cyclic structure of formula:
wherein K and L are a hydrogen or a lower alkyl group (preferably of 1 to about 8 carbon atoms), or optionally K and L can form an alicyclic hydrocarbon ring (preferably containing a maximum of 8 carbon atoms) or a heterocyclic ring (preferably containing a maximum of 6 carbon atoms; and having 1 to 2 heteroatoms selected from nitrogen, oxygen or sulfur);
R4 and R5 are each independently a hydrogen, a halogen (preferably chloro or fluoro), or a lower alkyl group (preferably of 1 to about 6 carbon atoms, more
preferably of 1 to 4 carbon atoms);
R6 is a hydrogen, a hydroxy group, oxygen (e.g., ketone), a nitrite ester, a nitrate ester, -OC(0)-CH2-B-D, wherein B is oxygen or sulfur, and D is NO or N02 , a nitroxylalkanoyl group (preferably of 2 to about 6 carbon atoms, more preferably of 2 to 4 carbon atoms), an alkoxy group (preferably of 1 to about 6 carbon atoms, more preferably of 1 to 4 carbon atoms), an alkylsilyloxy group (preferably of 3 to about 8 carbon atoms), a lower alkyl group (preferably of 1 to 6 carbon atoms, more preferably of 1 to 4 carbon atoms), wherein each of these groups is independently and optionally substituted with a hydroxy, a lower alkyl group, a lower alkenyl group, a lower alkynyl group, an alkoxy group, an amino group, a nitro group, a nitrile group, a carboxyl group, a haloalkyl group or -OCO-R, wherein Rg is as defined above;
R7 is a hydrogen, a hydroxy group, oxygen, nitrite ester, thionitrite ester, nitrate ester or thionitrate ester; R8 is a hydrogen or a halogen;
X is a lower alkyl group (preferably a methylene group), or sulfur if Rj is a haloalkyl group (preferably fluoromethyl); with the proviso that at least one of Rv R2, R3, R6 or R7is a nitrite, nitrate, thionitrite ester or thionitrate esterr A particularly preferred embodiment of the compound of formula (I) described herein is a compound of formula (I) wherein: (i) R is halogen; (ii) R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester; or (iii) R is halogen and R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester. More preferably, in the compound of formula (I), R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester; or R is halogen and R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester.
Another embodiment of the present invention describes preferred nitrosated and /or nitrosylated steroids and pharmaceutically acceptable salts thereof of formula (I) that are described herein as formula (II):
R.
(II)
wherein the dotted lines indicates a single or double bond;
R is a hydrogen or halogen;
Rα is a hydrogen, a hydroxy, a nitrite ester, a nitrate ester, a thionitrite ester, a thionitrate ester, a chloro, a sulfhydryl, N-methylpiperazin-1-yl, trimethylsilylmethyloxy, t-butyldimethylsilyoxy, a C1A alkyl group, or -OC(0)-Rn wherein Rn is propanoic acid, methyl or ethyl group;
R2 and R3 are each independently a hydrogen, a hydroxy group, a nitrite ester, a nitrate ester, methyl, a lower alkynyl group, or -OC(0)-R12 wherein R12is 2- furanyl, an ethoxy, a methyl, an ethyl, a propyl or a butyl group;
R2 and R3 may optionally form a cyclic structure of formula:
wherein K and L are a hydrogen, methyl or butyl, or optionally K and L can form a cyclopentyl or cyclohexyl ring); R4 and R5 are each independently a hydrogen, a chloro, a fluoro, or a methyl group;
Rg is a hydroxy group, oxygen, a nitrite ester, a nitrate ester, -OC(0)-CH2-B-D, wherein B is oxygen or sulfur and D is NO or N02 ;
R7 is a hydrogen, a hydroxy group, oxygen, nitrite ester, thionitrite ester, nitrate ester or thionitrate ester;
R8 is a hydrogen or a halogen, preferably chloro or bromo;
X is a methylene group; with the proviso that at least one of Rlr R2, R3, R6 or R7 is a nitrite, nitrate, thionitrite ester or thionitrate ester.
A particularly preferred embodiment of the compound of formula (II) described herein is a compound of formula (II) wherein: (i) R is halogen; (ii) R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester; or (iii) R is halogen and R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester. More preferably, in the compound of formula (II), R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester; or R is halogen and R7 is a nitrite ester, a thionitrite ester, a nitrate ester or a thionitrate ester.
Another embodiment of the present invention is nitrosated and /or nitrosylated steroids and pharmaceutically acceptable salts thereof of formula (III):
R 17
(III)
wherein the dotted lines indicates a single or double bond;
R13 is a hydrogen, a hydroxy group, a nitrite ester, a nitrate ester, thionitrite
ester, a thionitrate ester, a halogen (preferably chloro), a sulfhydryl group, a lower thioalkyl group (preferably of 1 to 4 carbon atoms), an alkoxy group (preferably of 1 to 6 carbon atoms, more preferably 1 to 4 carbon atoms), an acyloxy group (preferably of 2 to 6 carbon atoms, more preferably 2 to 4 carbon atoms), an alkylsilyloxy group (preferably of 3 to 8 carbon atoms, more preferably 3 to 6 carbon atoms), a lower alkyl group (preferably of 1 to 6 carbon atoms, more preferably of 1 to 4 carbon atoms), a heterocyclic group (preferably of 2 to 5 carbon atoms, more preferably 2 to 4 carbon atoms, and preferably having 1 to 2 hetero atoms), or an alicyclic hydrocarbon group (preferably of 3 to 6 carbon atoms), a lower alkenyl group, a lower alkynyl group;
R14 and R15 are each independently a hydrogen, a lower alkyl group (preferably of 1 to 8 carbon atoms, more preferably of 1 to 6 carbon atoms, most preferably a methyl, ethyl or propyl group), alicyclic group (preferably of 3 to 8 carbon atoms), and a heterocyclic group (preferably of 2 to 5 carbon atoms and 1 to 2 hetero atoms), a lower alkenyl group, a lower alkynyl group; optionally R14 and R15 taken together form an alicyclic group (preferably of 3 to 8 carbon atoms, more preferably 3 to 6 carbon atoms) or a heterocyclic group (preferably of 3 to 6 carbon atoms, more preferably of 2 to 4 carbon atoms; and having 1 to 2 hetero atoms);
R16 and R17are each independently a hydrogen, a halogen group (preferably a chloro, or a fluoro) or a lower alkyl group (preferably of 1 to 4 carbon atoms, more preferably of 1 to 3 carbon atoms, most preferably a methyl group), a lower alkenyl group, a lower alkynyl group;
R18 is a hydrogen, a hydroxy group, a nitrite ester, a nitrate ester, a thionitrite ester, a thionitrate ester or an acyloxy group (preferably of 2 to 4 carbon atoms, more preferably of 2 to 3 carbon atoms); with the proviso that R13 and/or R18 are a nitrite ester, a nitrate ester, a thionitrite ester or a thionitrate ester.
Another embodiment of the present invention describes compounds of formula (IV), and pharmaceutically acceptable esters, prodrugs and pharmaceutically acceptable salts thereof, wherein formula (IV) is:
A-B-C (IV)
wherein:
A is a residue of a hydroxyl-containing steroidal hormone. The hydroxyl- containing steroidal hormones include those described herein.
B is a linking group, preferably containing a maximum of 12 carbon atoms, connecting A through the hydroxyl moiety and C through the amino or hydroxyl group via an amide, ester, carbamate or carbonate linker.
C is an organic nitrite, nitrate, thionitrite or thionitrate compound or other nitric oxide donating compounds, such as furoxan derivatives. Representative examples of organic nitrite, nitrate, thionitrite and thionitrate compounds are glyceryl nitrate, amylnitrate, isosorbide mononitrate, isosorbide dinitrate, mannitaol nitrate, pentaerythritol nitrate, propatyl nitrate, S-nitrosoglutathione, S-nitroso-N- acetylcysteine, and the like. Additionally, C may be a nitric oxide donor such as those described herein.
Another embodiment of the present invention describes nitrosated and /or nitrosylated steroids and pharmaceutically acceptable salts thereof of formula (V), and pharmaceutically acceptable esters, prodrugs and pharmaceutically acceptable salts thereof, wherein formula (V) is:
R 23
(V)
wherein the dotted lines indicates a single or double bond;
R19 is a hydrogen, a hydroxy group, a nitrite ester, a nitrate ester, a thionitrite ester, a thionitrate ester, a halogen, a thiol group, an alkylmercapto group
(preferably of 1 to about 6 carbon atoms), a heterocyclic group (preferably of 2 to about 5 carbon atoms; and having 1 to 2 hetero atoms), an alkoxy group (preferably of 1 to about 6 carbon atoms), an alkylsilyloxy group (preferably of 3 to about 8 carbon atoms), a lower alkyl group (preferably of 1 to about 6 carbon atoms), wherein each of these groups is independently and optionally substituted with a hydroxy group, a lower alkyl group, a lower alkenyl group, a lower alkynyl group, an alkoxy group, an amino group, a nitro group, a nitrile group, a carboxyl group, a haloalkyl group, or -O O)-R9 wherein R, is as defined herein;
R20 and R2i are each independently a hydrogen, a hydroxy, a nitrite ester, a nitrate ester, a nitroxylalkanoyl group, a lower alkyl group (preferably of 1 to about 6 carbon atoms), a lower alkenyl group (preferably of 2 to about 6 carbon atoms), a lower alkynyl group (preferably of 2 to about 6 carbon atoms), an alkoxy group (preferably of 1 to about 6 carbon atoms), wherein each of these groups is independently and optionally substituted with a hydroxy, a lower alkyl group, a lower alkenyl group, a lower alkynyl group, an alkoxy group, an amino group, a nitro group, a nitrile group, a carboxy group, a haloalkyl group, or a group of formula -OC(O)-R10 or -SC(O)-R10 wherein R10 is 2-furanyl, a lower alkyl group (preferably of 1 to about 6 carbon atoms), a lower haloalkyl group or an alkoxy group (preferably 1 to about 6 carbon atoms); or R20 and R21 may optionally form a cyclic structure of formula:
wherein K and L are a hydrogen or a lower alkyl group (preferably of 1 to about 8 carbon atoms), or optionally K and L can form an alicyclic hydrocarbon ring (preferably containing a maximum of 8 carbon atoms) or a heterocyclic ring (preferably containing a maximum of 6 carbon atoms; and having 1 to 2 heteroatoms selected from nitrogen, oxygen or sulfur);
R22 and R23 are each independently a hydrogen, a halogen group (preferably chloro or fluoro) or a lower alkyl group (preferably of 1 to about 6 carbon atoms);
R24 is a hydrogen, a hydroxyl group or oxygen;
R25 is a hydrogen or a halogen group;
W is oxygen or sulfur;
Y is a covalent bond, methylene group, oxygen or an amino group;
Z is oxygen or an amino group; and n is an integer from 0 to 4.
Another embodiment of the present invention describes nitrosated and /or nitrosylated steroids and pharmaceutically acceptable salts thereof of formula (VI), and pharmaceutically acceptable esters, prodrugs and and pharmaceutically acceptable salts thereof, wherein formula (VI) is:
(VI)
wherein the dotted lines indicates a single or double bond;
R2ό is a hydrogen, a hydroxy group, a nitrite ester, a nitrate ester, a thionitrite ester, a thionitrate ester, a halogen, oxygen (e.g., a ketone), a thiol group, an alkylmercapto group (preferably of 1 to about 6 carbon atoms), a heterocyclic group (preferably of 2 to about 5 carbon atoms; and having 1 to 2 hetero atoms), an alkoxy group (preferably of 1 to about 6 carbon atoms), an alkylsilyloxy group (preferably of 3 to about 8 carbon atoms), a lower alkyl group (preferably of 1 to about 6 carbon atoms), wherein each of these groups is independently and optionally substituted with a hydroxy group, a lower alkyl group, a lower alkenyl group, a lower alkynyl
group, an alkoxy group, an amino group, a nitro group, a nitrile group, a carboxyl group, a haloalkyl group or -O O)-!^, wherein R9 is as defined herein; R20, R21, R22, R23, R24, R25, W, Y, Z and n are as defined herein.
The compounds of formulas (I), (II), (III), (IV), (V) and (VI) can be synthesized by one skilled in the art following the methods described herein, and by the methods described in, for example, U.S. Patent Nos. 5,707,984, 5,792,758, 5,837,698 and 5,985,862, the disclosures of each of which are incorporated by reference herein in their entirety.
The reactions are performed in solvents appropriate to the reagents, and materials used are suitable for the transformations being effected. One skilled in the art of organic synthesis will appreciate that the functionality present in the molecule must be consistent with the chemical transformation proposed. This will, on occasion, necessitate judgment by the routineer as to the order of synthetic steps, protecting groups required, and deprotection conditions. Substituents on the starting materials may be incompatible with some of the reaction conditions required in some of the methods described, but alternative methods and substituents compatible with the reaction conditions will be readily apparent to one skilled in the art. The use of sulfur and oxygen-protecting groups is well known in the art for protecting thiol, and alcohol groups against undesirable reactions during a synthetic procedure and many such protecting groups are known, including those described by Greene and Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, New York (1991).
Nitroso compounds of formula (I), (II) or (III) can be prepared by nitrosating or nitrosylating the parent steroid. Suitable O-nitrosating agents include a stoichiometric quantity of sodium nitrite in aqueous acid or fuming nitric acid and acetic anhydride or nitrosonium tetrafluoroborate in a suitable anhydrous solvent such as dichloromethane, THF, DMF, or acetonitrile with or without an amine base such as pyridine or triethylamine produce the compounds of formula (I), (II) or (III). Suitable S-nitrosylating agents include thionyl chloride such as thionyl chloride nitrite, thionyl dinitrite, a lower alkyl nitrite such as tert-butyl nitrite, or nitrosium tetrafluoroborate in a suitable anhydrous solvent such as methylene chloride, THF, DMF or acetonitrile produce the compounds of formula (I), (II) or (III).
Compounds of formula (IV) can be prepared following the methods
described in U.S. Patent No. 5,837,698, the disclosure of which is incorporated by reference herein in its entirety.
Nitroso compounds of formula (V) can be prepared by coupling the parent steroid with the appropriate nitrosated or nitrosylated compound by treatment with a dehydrating agent such as dicyclohexylcarbodiimide (DCC) or l-ethyl-3(3- dimethylaminopropyl) carbodiimide hydrochloride (EDACΗC1), with or without a catalyst, such as dimethylaminopyridine (DMAP) or 1-hydroxybenzotriazole (HOBt).
Nitroso compounds of formula (VI) can be prepared by coupling the parent steroid with the appropriate nitrosated or nitrosylated compound by treatment with a dehydrating agent such as DCC or EDAC.HC1. The nitrosated and/or nitrosylated steroid compounds of the present invention donate, transfer or release a biologically active form of nitrogen monoxide.
The compounds of the present invention include steroid compounds, such as those described herein, which have been nitrosylated through one or more sites such as oxygen (hydroxyl condensation), sulfur (sulfhydryl condensation), carbon and/or nitrogen. The nitrosated and /or nitrosylated steroid compounds of the present invention are capable of donating, transfering and /or releasing a biologically active form of nitrogen monoxide (i.e., nitric oxide). Nitrogen monoxide can exist in three forms: NO- (nitroxyl), NO* (uncharged nitric oxide) and NO+ (nitrosonium). NO* is a highly reactive short-lived species that is potentially toxic to cells. This is critical because the pharmacological efficacy of NO depends upon the form in which it is delivered. In contrast to the nitric oxide radical (NO*), nitrosonium (NO+) does not react with 02 or 02 ~ species, and functionalities capable of transferring and /or releasing NO+ and NO- are also resistant to decomposition in the presence of many redox metals. Consequently, administration of charged NO equivalents (positive and /or negative) is a more effective means of delivering a biologically active NO to the desired site of action.
The nitrosated and /or nitrosylated steroid compounds described herein can be used in combination with a nitric oxide donor (i.e., compounds that release nitric oxide or otherwise directly or indirectly deliver or transfer nitric oxide to a site of its activity, such as on a cell membrane, in vivo, and /or elevate or stimulate production of endogenous nitric oxide or EDRF in vivo and /or is a substrate for nitric oxide
synthase). "In combination," as used herein can mean that (i) the nitrosated and/or nitrosylated steroid and nitric oxide donor can be present together in the same composition; (ii) the nitrosated and /or nitrosylated steroid and nitric oxide donor can be administered separately; and /or (iii) the nitrosated and /or nitrosylated steroid and nitric oxide donor can be together in the form of a kit.
The term "nitric oxide" encompasses uncharged nitric oxide (NO*) and charged nitrogen monoxide species, preferably charged nitrogen monoxide species, such as nitrosonium ion (NO+) and nitroxyl ion (NO-). The reactive form of nitric oxide can be provided by gaseous nitric oxide. The nitrogen monoxide releasing, delivering or transferring compounds have the structure F-NO, wherein F is a nitrogen monoxide releasing, delivering or transferring moiety, and include any and all such compounds which provide nitrogen monoxide to its intended site of action in a form active for its intended purpose. The term "NO adducts" encompasses any nitrogen monoxide releasing, delivering or transferring compounds, including, for example, S-nitrosothiols, nitrites, nitrates, S-nitrothiols, sydnonimines, 2-hydroxy-2- nitrosohydrazines (NONOates), (E)-alkyl-2-[(E)-hydroxyimino]-5-nitro-3-hexene amines or amides, nitrosoamines, furoxans as well as substrates for the endogenous enzymes which synthesize nitric oxide. The "NO adducts" can be mono- nitrosylated, poly-nitrosylated, mono-nitrosated and /or poly-nitrosated or a combination thereof at a variety of naturally susceptible or artificially provided binding sites for biologically active forms of nitrogen monoxide.
One group of NO adducts is the S-nitrosothiols, which are compounds that include at least one -S-NO group. These compounds include S-nitroso-polypeptides (the term "polypeptide" includes proteins and polyarnino acids that do not possess an ascertained biological function, and derivatives thereof); S-nitrosylated amino acids (including natural and synthetic amino acids and their stereoisomers and racemic mixtures and derivatives thereof); S-nitrosylated sugars; S-nitrosylated, modified and unmodified, oligonucleotides (preferably of at least 5, and more preferably 5-200 nucleotides); straight or branched, saturated or unsaturated, aliphatic or aromatic, substituted or unsubstituted S-nitrosylated hydrocarbons; and S-nitroso heterocyclic compounds. S-nitrosothiols and methods for preparing them are described in U.S. Patent Nos. 5,380,758 and 5,703,073; WO 97/27749; WO 98/19672; and Oae et al, Org. Prep. Proc. Int., 25(3):165-198 (1983), the disclosures of
each of which are incorporated by reference herein in their entirety.
Another embodiment of the present invention is S-nitroso amino acids where the nitroso group is linked to a sulfur group of a sulfur-containing amino acid or derivative thereof. Such compounds include, for example, S-nitroso-N- acetylcysteine, S-nitroso-captopril, S-nitroso-N-acetylpenicillamine, S-nitroso- homocysteine, S-nitroso-cysteine and S-nitroso-glutathione.
Suitable S-nitrosylated proteins include thiol-containing proteins (where the NO group is attached to one or more sulfur groups on an amino acid or amino acid derivative thereof) from various functional classes including enzymes, such as tissue- type plasminogen activator (TPA) and cathepsin B; transport proteins, such as lipoproteins; heme proteins, such as hemoglobin and serum albumin; and biologically protective proteins, such as immunoglobulins, antibodies and cytokines. Such nitrosylated proteins are described in WO 93/09806, the disclosure of which is incorporated by reference herein in its entirety. Examples include polynitrosylated albumin where one or more thiol or other nucleophilic centers in the protein are modified.
Other examples of suitable S-nitrosothiols include: (i) HS(C(Re)(Rf))mSNO; (ii) ONS(C(Re)(Rf))mRe; and (iii) H2N-CH(C02H)-(CH2)m-C(0)NH-CH(CH2SNO)-C(0)NH-CH2-C02H; wherein m is an integer from 2 to 20; Re and Rf are each independently a hydrogen, an alkyl, a cycloalkoxy, a halogen, a hydroxy, a hydroxyalkyl, an alkoxyalkyl, an arylheterocyclic ring, a cycloalkylalkyl, a heterocyclicalkyl, an alkoxy, a haloalkoxy, an amino, an alkylamino, a dialkylamino, an arylamino, a diarylamino, an alkylarylamino, an alkoxyhaloalkyl, a haloalkoxy, a sulfonic acid, a sulfonic ester, an alkylsulfonic acid, an arylsulfonic acid, an arylalkoxy, an alkylthio, an arylthio, a cyano, an aminoalkyl, an aminoaryl, an aryl, an arylalkyl, an alkylaryl, a carboxamido, a alkyl carboxamido, an aryl carboxamido, an amidyl, a carboxyl, a carbamoyl, an alkylcarboxylic acid, an arylcarboxylic acid, an alkylcarbonyl, an arylcarbonyl, an ester, a carboxylic ester, an alkylcarboxylic ester, an arylcarboxylic ester, a sulfonamido, an alkylsulfonamido, an arylsulfonamido, a urea, a nitro, -T-Q, or (C(Re)(Rf))k-T-Q, or Re and Rf taken together are a carbonyl, a methanthial, a heterocyclic ring, a cycloalkyl group or a bridged cycloalkyl group; Q is -NO or -
N02; and T is independently a covalent bond, a carbonyl, an oxygen, -S(O)0- or - N(Ra)Rj-, wherein o is an integer from 0 to 2, Ra is a lone pair of electrons, a hydrogen or an alkyl group; R; is a hydrogen, an alkyl, an aryl, an alkylcarboxylic acid, an arylcarboxylic acid, an alkylcarboxylic ester, an arylcarboxylic ester, an alkylcarboxamido, an arylcarboxamido, an alkylaryl, an alkylsulfinyl, an alkylsulfonyl, an arylsulfinyl, an arylsulfonyl, a sulfonamido, a carboxamido, a carboxylic ester, an aminoalkyl, an aminoaryl, -CH2-C(T-Q)(Re)(Rf), or -(N202-)"*M+, wherein M+ is an organic or inorganic cation; with the proviso that when R; is -CH2- C(T-Q)(Re)(Rf) or -(N202-)*M+; then "-T-Q" can be a hydrogen, an alkyl group, an alkoxyalkyl group, an aminoalkyl group, a hydroxy group or an aryl group.
In cases where Re and Rf are a heterocyclic ring or taken together Re and Rf are a heterocyclic ring, then R; can be a substituent on any disubstituted nitrogen contained within the radical wherein R; is as defined herein.
Nitrosothiols can be prepared by various methods of synthesis. In general, the thiol precursor is prepared first, then converted to the S-nitrosothiol derivative by nitrosation of the thiol group with NaN02 under acidic conditions (pH is about 2.5) which yields the S-nitroso derivative. Acids which can be used for this purpose include aqueous sulfuric, acetic and hydrochloric acids. The thiol precursor can also be nitrosylated by reaction with an organic nitrite such as tert-butyl nitrite, or a nitrosonium salt such as nitrosonium tetraflurorborate in an inert solvent.
Another group of NO adducts for use in the present invention, where the NO adduct is a compound that donates, transfers or releases nitric oxide, include compounds comprising at least one ON-O-, ON-N- or ON-C- group. The compounds that include at least one ON-O-, ON-N- or ON-C- group are preferably ON-O-, ON-N- or ON-C-polypeptides (the term "polypeptide" includes proteins and polyamino acids that do not possess an ascertained biological function, and derivatives thereof); ON-O, ON-N- or ON-C-amino acids (including natural and synthetic amino acids and their stereoisomers and racemic mixtures); ON-O-, ON-N- or ON-C-sugars; ON-O-, ON-N- or ON-C- modified or unmodified oligonucleotides (comprising at least 5 nucleotides, preferably 5-200 nucleotides); ON-O-, ON-N- or ON-C- straight or branched, saturated or unsaturated, aliphatic or aromatic, substituted or unsubstituted hydrocarbons; and ON-O-, ON-N- or ON-C- heterocyclic compounds.
Another group of NO adducts for use in the present invention include nitrates that donate, transfer or release nitric oxide, such as compounds comprising at least one 02N-0-, 02N-N-, 02N-S- or 02N-C- group. Preferred among these compounds are 02N-0-, 02N-N-, 02N-S- or 02N-C- polypeptides (the term "polypeptide" includes proteins and also polyamino acids that do not possess an ascertained biological function, and derivatives thereof); 02N-0-, 02N-N-, 02N-S- or 02N-C- amino acids (including natural and synthetic amino acids and their stereoisomers and racemic mixtures); 02N-0-, 02N-N-, 02N-S- or 02N-C-sugars; 02N-0-, 02N-N-, 02N-S- or 02N-C- modified and unmodified oligonucleotides (comprising at least 5 nucleotides, preferably 5-200 nucleotides); 02N-0-, 02N-N-, 02N-S- or 02N-C- straight or branched, saturated or unsaturated, aliphatic or aromatic, substituted or unsubstituted hydrocarbons; and 02N-0-, 02N-N-, 02N-S- or 02N-C- heterocyclic compounds. Preferred examples of compounds comprising at least one 02N-0-, 02N-N-, 02N-S- or 02N-C- group include isosorbide dinitrate, isosorbide mononitrate, clonitrate, erythrityltetranitrate, mannitol hexanitrate, nitroglycerin, pentaerythritoltetranitrate, pentrinitrol and propatylnitrate.
Another group of NO adducts are N-oxo-N-nitrosoamines that donate, transfer or release nitric oxide and are represented by the formula: R1R2-N(0-M+)- NO, where R1 and R2 are each independently a polypeptide, an amino acid, a sugar, a modified or unmodified oligonucleotide, a straight or branched, saturated or unsaturated, aliphatic or aromatic, substituted or unsubstituted hydrocarbon, or a heterocyclic group, and where M+ is an organic or inorganic cation, such as, for example, an alkyl substituted ammonium cation or a Group I metal cation.
Another group of NO adducts are thionitrates that donate, transfer or release nitric oxide and are represented by the formula: Rα-(S)-N02, where R1 is a polypeptide, an amino acid, a sugar, a modified or unmodified oligonucleotide, a straight or branched, saturated or unsaturated, aliphatic or aromatic, substituted or unsubstituted hydrocarbon, or a heterocyclic group. Preferred are those compounds where R1 is a polypeptide or hydrocarbon with a pair or pairs of thiols that are sufficiently structurally proximate, i.e., vicinal, that the pair of thiols will be reduced to a disulfide. Compounds which form disulfide species release nitroxyl ion (NO-) and uncharged nitric oxide (NO*). Compounds where the thiol groups are not sufficiently close to form disulfide bridges generally provide nitric oxide as the NO-
form and not as the uncharged NO* form.
The present invention is also directed to compounds that stimulate endogenous NO or elevate levels of endogenous endothelium-derived relaxing factor (EDRF) in vivo or are substrates for nitric oxide synthase. Such compounds include, for example, L-arginine, L-homoarginine, and N-hydroxy-L-arginine, including their nitrosated and nitrosylated analogs (e.g., nitrosated L-arginine, nitrosylated L-arginine, nitrosated N-hydroxy-L-arginine, nitrosylated N-hydroxy- L-arginine, nitrosated L-homoarginine and nitrosylated L-homoarginine), precursors of L-arginine and /or physiologically acceptable salts thereof, including, for example, citruUine, ornithine, glutamine, lysine, polypeptides comprising these amino acids, inhibitors of the enzyme arginase (e.g., N-hydroxy-L-arginine and 2(S)-amino-6- boronohexanoic acid) and the substrates for nitric oxide synthase, cytokines, adenosin, bradykinin, calreticulin, bisacodyl, and phenolphthalein. EDRF is a vascular relaxing factor secreted by the endothelium, and has been identified as nitric oxide (NO) or a closely related derivative thereof (Palmer et al, Nature, 327:524-526 (1987); Ignarro et al, Proc. Natl. Acad. Sci. USA, 84:9265-9269 (1987)). In preventing and /or treating cardiovascular diseases and disorders, the nitrosated and/or nitrosylated steroids and, optionally, at least one compound that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase can be administered directly to the damaged vascular surface intravenously by using an intraarterial or intravenous catheter, suitable for delivery of the compounds to the desired location. The location of damaged arterial surfaces is determined by conventional diagnostic methods, such as X-ray angiography, performed using routine and well-known methods available to one skilled in the art. In addition, administration of the nitrosated and /or nitrosylated steroids, and, optionally, NO donors, using an intraarterial or intravenous catheter is performed using routine methods well known to one skilled in the art. Typically, the compound or composition is delivered to the site of angioplasty through the same catheter used for the primary procedure, usually introduced to the carotid or coronary artery at the time of angioplasty balloon inflation. The nitrosated and /or nitrosylated steroids, and, optionally, NO donors, slowly decompose at body temperature over a prolonged period of time releasing nitric oxide at a rate effective to prevent and /or
treat cardiovascular diseases and disorders including, for example, restenosis.
The compounds and compositions of the present invention can be administered by any available and effective delivery system including, but not limited to, orally, bucally, parenterally, by inhalation spray, by topical application, by injection, transdermally, or rectally (e.g., by the use of suppositories) in dosage unit formulations containing conventional nontoxic pharmaceutically acceptable carriers, adjuvants, and vehicles, as desired. Parenteral includes subcutaneous injections, intravenous, intramuscular, intrasternal injection, or infusion techniques. Transdermal compound administration, which is known to one skilled in the art, involves the delivery of pharmaceutical compounds via percutaneous passage of the compound into the systemic circulation of the patient. Topical administration can also involve the use of transdermal administration such as transdermal patches or iontophoresis devices. Other components can be incorporated into the transdermal patches as well. For example, compositions and/or transdermal patches can be formulated with one or more preservatives or bacteriostatic agents including, but not limited to, methyl hydroxybenzoate, propyl hydroxybenzoate, chlorocresol, benzalkonium chloride, and the like. Dosage forms for topical administration of the compounds and compositions can include creams, sprays, lotions, gels, ointments, eye drops, nose drops, ear drops, and the like. In such dosage forms, the compositions of the invention can be mixed to form white, smooth, homogeneous, opaque cream or lotion with, for example, benzyl alcohol 1% or 2% (wt/wt) as a preservative, emulsifying wax, glycerin, isopropyl palmitate, lactic acid, purified water and sorbitol solution. In addition, the compositions can contain polyethylene glycol 400. They can be mixed to form ointments with, for example, benzyl alcohol 2% (wt/wt) as preservative, white petrolatum, emulsifying wax, and tenox II (butylated hydroxyanisole, propyl gallate, citric acid, propylene glycol). Woven pads or rolls of bandaging material, e.g., gauze, can be impregnated with the compositions in solution, lotion, cream, ointment or other such form can also be used for topical application. The compositions can also be applied topically using a transdermal system, such as one of an acrylic-based polymer adhesive with a resinous crosslinking agent impregnated with the composition and laminated to an impermeable backing.
Solid dosage forms for oral administration can include capsules, tablets,
effervescent tablets, chewable tablets, pills, powders, sachets, granules and gels. In such solid dosage forms, the active compounds can be admixed with at least one inert diluent such as sucrose, lactose or starch. Such dosage forms can also comprise, as in normal practice, additional substances other than inert diluents, e.g., lubricating agents such as magnesium stearate. In the case of capsules, tablets, effervescent tablets, and pills, the dosage forms can also comprise buffering agents. Soft gelatin capsules can be prepared to contain a mixture of the active compounds or compositions of the present invention and vegetable oil. Hard gelatin capsules can contain granules of the active compound in combination with a solid, pulverulent carrier such as lactose, saccharose, sorbitol, mannitol, potato starch, corn starch, amylopectin, cellulose derivatives of gelatin. Tablets and pills can be prepared with enteric coatings.
Liquid dosage forms for oral administration can include pharmaceutically acceptable emulsions, solutions, suspensions, syrups, and elixirs containing inert diluents commonly used in the art, such as water. Such compositions can also comprise adjuvants, such as wetting agents, emulsifying and suspending agents, and sweetening, flavoring, and perfuming agents.
Suppositories for vaginal or rectal administration of the compounds and compositions of the invention can be prepared by mixing the compounds or compositions with a suitable nonirritating excipient such as cocoa butter and polyethylene glycols which are solid at room temperature but liquid at bodytemperature, such that they will melt and release the drug.
Injectable preparations, for example, sterile injectable aqueous or oleaginous suspensions can be formulated according to the known art using suitable dispersing agents, wetting agents and/or suspending agents. The sterile injectable preparation can also be a sterile injectable solution or suspension in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that can be used are water, Ringer's solution, and isotonic sodium chloride solution. Sterile fixed oils are also conventionally used as a solvent or suspending medium.
The compositions of this invention can further include conventional excipients, i.e., pharmaceutically acceptable organic or inorganic carrier substances suitable for parenteral application which do not deleteriously react with the active
compounds. Suitable pharmaceutically acceptable carriers include, for example, water, salt solutions, alcohol, vegetable oils, polyethylene glycols, gelatin, lactose, amylose, magnesium stearate, talc, surfactants, silicic acid, viscous paraffin, perfume oil, fatty acid monoglycerides and diglycerides, petroethral fatty acid esters, hydroxymethyl-cellulose, polyvinylpyrrolidone, and the like. The pharmaceutical preparations can be sterilized and if desired, mixed with auxiliary agents, e.g., lubricants, preservatives, stabilizers, wetting agents, emulsifiers, salts for influencing osmotic pressure, buffers, colorings, flavoring and /or aromatic substances and the like which do not deleteriously react with the active compounds. For parenteral application, particularly suitable vehicles consist of solutions, preferably oily or aqueous solutions, as well as suspensions, emulsions, or implants. Aqueous suspensions may contain substances which increase the viscosity of the suspension and include, for example, sodium carboxymethyl cellulose, sorbitol and/or dextran. Optionally, the suspension may also contain stabilizers. The composition, if desired, can also contain minor amounts of wetting agents, emulsifying agents and /or pH buffering agents. The composition can be a liquid solution, suspension, emulsion, tablet, pill, capsule, sustained release formulation, or powder. The composition can be formulated as a suppository, with traditional binders and carriers such as triglycerides. Oral formulations can include standard carriers such as pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharine, cellulose, magnesium carbonate, and the like.
Various delivery systems are known and can be used to administer the compounds or compositions of the present invention, including, for example, encapsulation in liposomes, microbubbles, emulsions, microparticles, microcapsules and the like. The required dosage can be administered as a single unit or in a sustained release form.
The bioavailabilty of the compositions can be enhanced by micronization of the formulations using conventional techniques such as grinding, milling, spray drying and the like in the presence of suitable excipients or agents such as phospholipids or surfactants.
The compounds and compositions of the present invention can be formulated as pharmaceutically acceptable salts. Pharmaceutically acceptable salts include, for
example, alkali metal salts and addition salts of free acids or free bases. The nature of the salt is not critical, provided that it is pharmaceutically-acceptable. Suitable pharmaceutically-acceptable acid addition salts may be prepared from an inorganic acid or from an organic acid. Examples of such inorganic acids include, but are not limited to, hydrochloric, hydrobromic, hydroiodic, nitrous (nitrite salt), nitric (nitrate salt), carbonic, sulfuric, phosphoric acid, and the like. Appropriate organic acids include, but are not limited to, aliphatic, cycloaliphatic, aromatic, heterocyclic, carboxylic and sulfonic classes of organic acids, such as, for example, formic, acetic, propionic, succinic, glycolic, gluconic, lactic, malic, tartaric, citric, ascorbic, glucuronic, maleic, fumaric, pyruvic, aspartic, glutamic, benzoic, anthranilic, mesylic, salicylic, p-hydroxybenzoic, phenylacetic, mandelic, embonic (pamoic), methanesulfonic, ethanesulfonic, benzenesulfonic, pantothenic, toluenesulfonic, 2- hydroxyethanesuifonic, sulfanilic, stearic, algenic, β-hydroxybutyric, cyclohexylaminosulfonic, galactaric and galacturonic acid and the like. Suitable pharmaceutically-acceptable base addition salts include, but are not limited to, metallic salts made from aluminum, calcium, lithium, magnesium, potassium, sodium and zinc or organic salts made from primary, secondary and tertiary amines, cyclic amines, N,N'-dibenzylethylenediamine, chloroprocaine, choline, diethanolamine, ethylenediamine, meglumine (N-methylglucamine) and procaine and the like. All of these salts may be prepared by conventional means from the corresponding compound by reacting, for example, the appropriate acid or base with the compound.
The term "therapeutically effective amount," for the purposes of the invention, refers to the amount of the nitric oxide releasing compound (e.g., nitrosated and /or nitrosylated steroid and /or nitric oxide donor) that is effective to achieve its intended purpose. The methods of treatment (including alleviation and amelioration) involve administering a therapeutically effective amount of the nitric oxide releasing compounds described herein after the onset of cardiovascular diseases or disorders, whereas the methods of prevention involve administering a therapeutically effective amount of the nitric oxide releasing compounds described herein prior to or at the onset of the cardiovascular diseases or disorders.
While individual needs may vary, determination of optimal ranges for effective amounts of each nitric oxide releasing compound is within the skill of the
art. Generally, the dosage required to provide an effective amount of the compounds and compositions, which can be adjusted by one of ordinary skill in the art, will vary depending on the age, health, physical condition, sex, diet and medical condition of the patient, the severity of the cardiovascular disease, the route of administration, pharmacological considerations such as the activity, efficacy, pharmacokinetic and toxicology profiles of the particular compound used, whether a drug delivery system is used, and whether the compound is administered as part of a drug combination. Thus, the dosage regimen actually used can vary widely and therefore may deviate from the preferred dosage regimen set forth herein. Preparations which are suitable for treatment of artificial surfaces, such as a medical device, instrument and endothelium are used in concentrations of about 500-700 mM of compound delivered by sterile drip infusion in a physiological liquid over 2-3 minute periods in amounts of 2-3 ml per 25 kg body weight. Intravenous dosages are about 0.01 to 10 mg/Kg of body weight/day and more preferably 0.05 to 5.0 mg/Kg of body weight/day.
Another embodiment of the invention provides compositions comprising at least one nitrosylated steroid, and, optionally, at least one compound that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase, bound to a matrix. Preferably, the nitrosated and/or nitrosylated steroid is of formula (I), formula (II), formula (III), formula (IV), formula (V) or formula (VI). Preferably, the compounds that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase (i.e., NO donors) are those described herein. The nitrosated and /or nitrosylated steroids, and, optionally, NO donors, can be incorporated into a natural or synthetic matrix which can then be applied with specificity to a biological site of interest. Accordingly the steroid and, optionally, NO donor is "bound to the matrix" which means that the nitrosated or nitrosylated steroids, and, optionally, NO donors, are physically and /or chemically associated with part of, incorporated with, attached to, or contained within the natural or synthetic matrix. Physical association or bonding can be achieved, for example, by coprecipitation of the nitrosated and /or nitrosylated steroid, and, optionally, NO donor, with the matrix. Chemical association or bonding can be achieved by, for
example, covalent bonding of a nucleophillic moiety of the nitrosated and/or nitrosylated steroid, and, optionally, NO donor, to the matrix, such that the steroid is part of the matrix itself. The manner in which the nitrosated and /or nitrosylated steroid, and, optionally, NO donor, is associated, part of, attached to, incorporated with or contained within (i.e. "bound to") the matrix is inconsequential to the present invention and all means of association, incorporation, attachment, and bonding are contemplated herein. Incorporation of the nitrosated and /or nitrosylated steroids, and, optionally, NO donors, into the matrix results in site-specific application, thereby enhancing selectivity of action for the released nitric oxide and parent steroid. Additionally, incorporation of the nitrosated and /or nitrosylated steroids into the matrix reduces the rate of release of the nitric oxide and the parent steroid. This prolongs the release of the nitric oxide and the parent steroid thereby allowing for efficient dosing to achieve a desired biological effect so that the frequency of dosing can be reduced. Any of a wide variety of natural or synthetic polymers can be used as the matrix in the context of the present invention. It is only necessary for the matrix to be biologically acceptable. Exemplary matrixes suitable for use in the present invention are polymers including, for example, polyolefins (such as polystyrene, polypropylene, polyethylene, high density polyethylene, polytetrafluorethylene, polyvinylidene diflouride and polyvinylchloride), polyethylenimine or derivatives thereof, polyethers (such as polyethylene glycol), polyesters (such as poly-L-lactic acid, poly-D, L-lactic, poly-D-lactic, polyglycolic, poly-(lactide/glycolide)), polyanhydrides, polyhydroxybutyrates, polyamides (such as nylon), polyurethanes, polyurethane copolymers (such as pellethane polymers), polyacrylates (such as polymethacrylate, poly (2-(methacryloyloxyethyl)-2'-(trimethylammonium)ethyl phosphate inner salt-co-n-dodecyl methacrylate), mixtures of polymers (such as polylactic acid/polylysine copolymers, polyurethane/polyester copolymers, polyurethane/polyether copolymers, nylon/polyether copolymers, such as vestamid), biopolymers (such as peptides, proteins, oligonucleotides, antibodies, peptide hormones, glycoproteins, glycogen and nucleic acids), starburst dendrimers, natural fibrous matrix (such as filter paper), synthetic fibrous matrix materials (such as three-dimensional lattice of synthetic polymers and copolymers) and the like. Exemplary polymers are described in U. S. Patent Nos. 5,705,583, 5,770,645 and
5,994,444 and co-pending Application Serial No. 08/460,465, the disclosures of which are incorporated by reference herein in their entirety.
The physical and structural characteristics of the matrixes suitable for use in the present invention are not critical, but depend on the application. It will be appreciated by one skilled in the art that where the matrix-steroid composition of the present invention are intended for local, relatively short term administration or similar administration they need not be biodegradable. For some uses, such as postangioplasty, coronary bypass surgery or intimal hyperplasia associated with vascular graft implants or the like, it may be desirable for the matrix to slowly dissolve in a physiological environment or to be biodegradable.
The nitrosated and/or nitrosylated steroid, and, optionally, at least one compound that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase, bound to the matrix may be administered in a wide variety of forms or delivery means. Any delivery means should adequately protect the integrity of the nitric oxide prior to its release and should control the release of the nitric oxide at such a rate, in such an amount, and in such a location as to serve as an effective means for prevention and /or treatment of cardiovascular diseases and disorders, including restenosis. Delivery means for local administration include, for example, sutures, vascular implants, stents, heart valves, drug pumps, drug delivery catheters and the like. Delivery means for systemic administration include, for example, solutions, suspensions, emulsions, capsules, powders, sachets, tablets, effervescent tablets, topical patches, lozenges, aerosols, liposomes, microparticles, microspheres, beads and the like. The matrix itself may be structurally sufficient to serve as a delivery means.
The nitrosated and /or nitrosylated steroid, and, optionally, at least one compound that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase, bound to the matrix can also be used to coat the surface of a medical device or instrument that comes into contact with blood (including blood components and blood products) or vascular tissue thereby rendering the surface passive. U.S. Patent Nos. 5,837,008, 5,665,077 and 5,797,887, the disclosures of each of which are incorporated by reference herein in their entirety, describe methods for
coating a surface of a medical device or instrument. Thus, for example, (i) all or a portion of the medical device may be coated with the nitrosated and /or nitrosylated steroids, and, optionally, NO donors, either as the coating per se or bound to a matrix, as described herein; (ii) all or a portion of the medical device may be produced from a material which includes the nitrosated and /or nitrosylated steroid, and, optionally, NO donor, per se or bound to a matrix, as described herein.
It is also contemplated that artificial surfaces will vary depending on the nature of the surface, and such characteristics including contour, crystallinity, hydrophobicity, hydrophilicity, capacity for hydrogen bonding, and flexibility of the molecular backbone and polymers. Therefore, using routine methods, one of ordinary skill will be able to customize the coating technique by adjusting such parameters as the amount of adduct, length of treatment, temperature, diluents, and storage conditions, in order to provide optimal coating of each particular type of surface. After the device or artificial material has been coated with the nitrosated and /or nitrosylated steroid and, optionally, NO donor, it will be suitable for its intended use, including, for example, implantation as a heart valve, insertion as a catheter, insertion as a stent, or for cardiopulmonary oxygenation or hemodialysis. Another embodiment of the present invention provides methods for the prevention of platelet aggregation and platelet adhesion caused by the exposure of blood (including blood components or blood products) to a medical device or instrument by incorporating at least one nitrosated and/or nitrosylated steroid, and, optionally, at least one compound that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase, capable of releasing a therapeutically effective amount of nitric oxide, into and /or on the portion(s) of the medical device that come into contact with blood (including blood components or blood products) or vascular tissue. The steroids, and, optionally, NO donors, may be directly or indirectly linked to the natural or synthetic polymeric material from which all or a portion of the device is made, as disclosed in co-pending Application Serial No. 08/460,465, the disclosure of which is incorporated by reference herein in its entirety. Alternatively, the nitrosated and /or nitrosylated steroids, and, optionally, NO donors, may be incorporated into the body of the device which is formed of a biodegradable or
bioresorbable material, including the matrix described herein. Thus the nitric oxide is released over a sustained period of the resorption or degradation of the body of the device.
The present invention also describes methods for the administration of nitrosated and /or nitrosylated steroids, and, optionally, at least one compound that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase, in combination with one or more therapeutic substances for the prevention and /or treatment of cardiovascular diseases and disorders including, for example, restenosis. Therapeutic substances used in the present invention may be any therapeutic substance which possesses desirable therapeutic characteristics for the application to a blood vessel. This can include both solid substances and liquid substances. Examples of therapeutic substances include, for example, heparin, hirudin, hyaluronic acid, vasoconstrictors, vasodilators, cellular adhesion promotors, tocopherol, angiopeptin, aspirin, ACE inhibitors, growth factors, oligonucleotides, and more generally, antiplatelet agents, anticoagulant agents, antimitotic agents, antioxidants, antimetabolite agents and anti-inflammatory agents. Antiplatelet agents can include drugs such as aspirin and dipyridamole. Anticoagulant agents can include drugs such as heparin, coumadin, protamine, hirudin and tick anticoagulant protein. Antimitotic agents and antimetabolite agents can include drugs such as methotrexate, azathioprine, vincristine, vinblastine, fluorouracil, adriamycin and mutamycin.
Another embodiment of the present invention relates to local administration of the nitrosated and /or nitrosylated steroids, and, optionally, at least one compound that donates, transfers or releases nitric oxide and /or stimulates the endogenous production of NO or EDRF in vivo and /or is a substrate for nitric oxide synthase, to the site of injured or damaged tissue (e.g., damaged blood vessels) for the treatment of the injured or damaged tissue. Such damage may result from the use of a medical device in an invasive procedure. Thus, for example, in treating blocked vasculature by, for example, angioplasty, damage can result to the blood vessel. Such damage may be treated by use of the nitrosated and/or nitrosylated steroids described herein. In addition to repair of the damaged tissue, such treatment can also be used to prevent and /or alleviate and /or delay re-occlusions,
for example, restenosis. Preferably, all or most of the damaged area is coated with the nitrosated and /or nitrosylated steroids described herein per se or in a pharmaceutically acceptable carrier or excipient which serves as a coating matrix, including the matrix described herein. This coating matrix can be of a liquid, gel or semisolid consistency. The nitrosated and/or nitrosylated steroids can be applied in combination with one or more therapeutic agents, such as those listed above. The carrier or matrix can be made of or include agents which provide for metered or sustained release of the therapeutic agents.
EXAMPLES The following non-limiting examples further describe and enable one of ordinary skill in the art to make and use the present invention. Example 1: 9 -fluoro-16 -methyl-llβ,17 /21-trihydroxypregna-l,4-diene-3,20- dione-21-nitrate ester (dexamethasone C-21 nitrate ester) A mixture of acetic anhydride (2 mL) and fuming nitric acid (density =1.5 g/mL, 0.5 mL) was cooled over ice/ salt and a precooled suspension of dexamethasone (Sigma Chemical Company, 1 g, 2.55 mmol) in CHC13 (10 mL) added slowly. After stirring for 2 hr over ice, the crude reaction mixture was diluted with dichloromethane and washed carefully with saturated sodium bicarbonate. The organic phase was dried over sodium sulphate, filtered and evaporated. The crude reaction mixture was chromatographed twice on silica gel (40 micron, Baker; eluent, ethyl acetate: dichloromethane 1:3) to give the product as a white solid (100 mg). l NMR (THF-d8): δ 7.28 (d, j=10 Hz, 1H), 6.24 (dd, j=10 Hz, 1.4 Hz, 1H), 6.05 (s, 1H), 5.40 (dd, j=43 Hz, 19 Hz, 2H), 4.75 (s, 2H), 4.37 (d, j=10 Hz, 1H), 3.06-3.19 (m, 1H), 2.28-2.80 (m, 5H), 1.75-1.97 (m, 2H), 1.51-1.70 (m, 2H), 1.65 (s, 3H), 1.22-1.32 (m, 1H), 1.11 (s, 3H), 0.95 (d, j=7 Hz, 3H). 13C NMR (THF-d8): δ 201.26, 183.19, 163.70, 149.70, 127.98, 123.21, 100.15, 97.83, 89.79, 73.80, 70.03, 69.81, 47.27, 46.90, 46.72, 42.51, 34.95, 34.87, 32.96, 32.88, 30.64, 29.23, 26.07, 21.41, 14.55, 12.62.
Example 2: llβ,17α,21-trihydroxy-l,4-pregnadiene-3, 20-dione 21-nitrate ester Fuming nitric acid (0.25 mL, 5.91 mmol) was added to acetic anhydride (1 mL) at -10<C and stirred for 5 min. A suspension of prednisolone (Sigma Chemical Company , 0.52 g, 1.44 mmol) in anhydrous chloroform (10 mL) was added to the resulting solution and stirred at -10<C for 1 h. The reaction was quenched with water (50 mL). The resulting white solid was filtered off and washed with
chloroform (100 mL) then dry under vacuum to obtain 0.456 g (78 % yield) of a white solid. Η-NMR (d6-OMSO): δ 0.81 (s, 3H, C-18), 1.39 (s, 3H, C-19), 4.30 (s, 1H, C-ll), 5.40 (AB , 2 H, C-21), 5.92 (s, 1H, C-4), 6.17 (d, 1H, / = 10 Hz, C-2), 7.33 (d, 1 H, / = 10 Hz, C-l); 13C-NMR (d6-OMSO): δ 6.48, 20.89, 23.52, 30.93, 31.36, 33.35, 33.96, 38.7, 43.78, 47.52, 51.09, 55.32, 68.27, 75.45, 88.66, 121.63, 127.09, 156.66, 170.45, 185.19, 204.55; MS: 406.2 (M+l).
Example 3: Biological Response of Human Coronary Artery Smooth Muscle Cells (CASMC) to Steroid Nitrates and Their Non-Nitrated Parent Molecules Vascular Smooth Muscle Cell (SMC) Antiproliferation Assay
The cells used in this assay were human coronary artery smooth muscle cells (CASMC) supplied by Clonetics Corp. (San Diego, CA). They were maintained in SmGM-2 growth medium (Clonetics Corp.), which consisted of modified MCDB 131 medium supplemented with 5% (v/v) fetal bovine serum (FBS), 0.5 ng/mL human recombinant epidermal growth factor (EGF), 2 ng/mL human recombinant fibroblast growth factor (FGF), 5 μg/mL bovine insulin, 50 μg/mL gentamicin sulfate, and 50 ng/mL amphotericin B under humidified 95% air-5% C02 at 37°C. Cells were used for experiments up to about 17 cumulative population doublings and still stained positive for smooth muscle -actin (i.e., usually by passage 9). For the SMC antiproliferation assay, the cells were seeded at 3 x 104 viable cells in 2 mL of SmGM-2 medium per well of a Corning 24 well plate (Corning, NY). Stock solutions of the steroid test compounds (with or without a nitric oxide adduct) were prepared just prior to addition to the cells, by dissolving in ethanol at a concentration of 50 mM. This stock solution was diluted, as required, with ethanol (usually 1:2 serially to yield diluted stock solutions of 25, 12.5, 6.25, and 3.125 mM, respectively). On the same day, but after the cells had attached and spread out (about 3 hr), each test compound (2 FL from appropriate, diluted stock solutions) was added to four replicate wells for each concentration (final concentrations of 50, 25, 12.5, 6.25, and 3.125 μM). Control cultures received 2 FL of ethanol per well (n=4). On the following morning, the cultures were examined microscopically and their condition recorded. On the third day after test compound addition (-68 hr), the cultures were examined microscopically again and the viable cells counted with an hemacytometer following trypsinization with 0.25% trypsin-lmM EDTA. Trypan
Blue dye exclusion was used to discriminate between viable and dead cells. The results were usually presented as % of the control viable cell count (mean±SEM).
The proliferation of vascular smooth muscle cells, including those obtained from human CASMC, was inhibited by nitric oxide. In the antiproliferation assay, CASMC were exposed to increasing concentrations of the NO-donor containing steroid and the parent steroid. On the day after cell seeding and introduction of the steroids, the NO-donor containing steroid-treated cultures showed extensive cell death by microscopic observation and LDH (lactate dehydrogenase) release. When the cells were counted on Day 3 of the assay, this was confirmed. The highest concentrations of the NO-donor containing steroid resulted in few or no viable cells, indicating not only the suppression of proliferation, but also the destruction of the initial seeding inoculum of cells. The parent steroid, even at very high concentrations, suppressed cell proliferation (the number of viable cells was reduced versus the control cultures) but did not cause cell death, as evidenced by the recovery of viable cells and the absence of LDH released. These results are shown in Fig. 1 and Fig. 2. In particular, Fig. 1 shows the dose response curve of Coronary Artery Smooth Muscle Cells of the compound from Example 1 (i.e 9 -fluoro-16α- methyl-llβ,17 ,21-trihydroxypregna-l,4-diene-3,20-dione-21-nitrate ester) and the parent steroid, dexamethasone. The steroid nitrate ester was unexpectedly superior relative to the parent steroid in inhibiting the proliferation of vascular smooth muscle cells. Fig. 2 shows the dose response curve of Coronary Artery Smooth Muscle Cells to the compound of Example 2 (i.e., llβ,17α,21-trihydroxy-l,4- pregnadiene-3,20-dione 21-nitrate ester) and the parent steroid, prednisolone. The steroid nitrate ester was unexpectedly superior relative to the parent steroid in inhibiting the proliferation of vascular smooth muscle cells.
The disclosure of each patent, patent application and publication cited or described in the specification is hereby incorporated by reference herein in its entirety.
Although the invention has been set forth in detail, one skilled in the art will appreciate that numerous changes and modifications may be made without departing from the spirit and scope of the present invention.