CN101272750A - 混合脊柱融合椎间植入体 - Google Patents
混合脊柱融合椎间植入体 Download PDFInfo
- Publication number
- CN101272750A CN101272750A CNA2006800352555A CN200680035255A CN101272750A CN 101272750 A CN101272750 A CN 101272750A CN A2006800352555 A CNA2006800352555 A CN A2006800352555A CN 200680035255 A CN200680035255 A CN 200680035255A CN 101272750 A CN101272750 A CN 101272750A
- Authority
- CN
- China
- Prior art keywords
- implant
- intervertebral implant
- expansion element
- horizontal expansion
- supporter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/4455—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages
- A61F2/4465—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages having a circular or kidney shaped cross-section substantially perpendicular to the axis of the spine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30721—Accessories
- A61F2/30728—Collars; Bone edge protectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/46—Special tools or methods for implanting or extracting artificial joints, accessories, bone grafts or substitutes, or particular adaptations therefor
- A61F2/4603—Special tools or methods for implanting or extracting artificial joints, accessories, bone grafts or substitutes, or particular adaptations therefor for insertion or extraction of endoprosthetic joints or of accessories thereof
- A61F2/4611—Special tools or methods for implanting or extracting artificial joints, accessories, bone grafts or substitutes, or particular adaptations therefor for insertion or extraction of endoprosthetic joints or of accessories thereof of spinal prostheses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A61F2002/2817—Bone stimulation by chemical reactions or by osteogenic or biological products for enhancing ossification, e.g. by bone morphogenetic or morphogenic proteins [BMP] or by transforming growth factors [TGF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A61F2002/2835—Bone graft implants for filling a bony defect or an endoprosthesis cavity, e.g. by synthetic material or biological material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/30004—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis
- A61F2002/30014—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis differing in elasticity, stiffness or compressibility
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/30004—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis
- A61F2002/30056—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis differing in radiographic density
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/3006—Properties of materials and coating materials
- A61F2002/30062—(bio)absorbable, biodegradable, bioerodable, (bio)resorbable, resorptive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/3006—Properties of materials and coating materials
- A61F2002/3008—Properties of materials and coating materials radio-opaque, e.g. radio-opaque markers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30112—Rounded shapes, e.g. with rounded corners
- A61F2002/30125—Rounded shapes, e.g. with rounded corners elliptical or oval
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30112—Rounded shapes, e.g. with rounded corners
- A61F2002/30133—Rounded shapes, e.g. with rounded corners kidney-shaped or bean-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30138—Convex polygonal shapes
- A61F2002/30153—Convex polygonal shapes rectangular
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30138—Convex polygonal shapes
- A61F2002/30158—Convex polygonal shapes trapezoidal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/302—Three-dimensional shapes toroidal, e.g. rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30224—Three-dimensional shapes cylindrical
- A61F2002/3023—Three-dimensional shapes cylindrical wedge-shaped cylinders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2002/30331—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements made by longitudinally pushing a protrusion into a complementarily-shaped recess, e.g. held by friction fit
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2002/30476—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements locked by an additional locking mechanism
- A61F2002/305—Snap connection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30535—Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30593—Special structural features of bone or joint prostheses not otherwise provided for hollow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30535—Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30604—Special structural features of bone or joint prostheses not otherwise provided for modular
- A61F2002/30616—Sets comprising a plurality of prosthetic parts of different sizes or orientations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30667—Features concerning an interaction with the environment or a particular use of the prosthesis
- A61F2002/30677—Means for introducing or releasing pharmaceutical products, e.g. antibiotics, into the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/30772—Apertures or holes, e.g. of circular cross section
- A61F2002/30784—Plurality of holes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/30878—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves with non-sharp protrusions, for instance contacting the bone for anchoring, e.g. keels, pegs, pins, posts, shanks, stems, struts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2002/448—Joints for the spine, e.g. vertebrae, spinal discs comprising multiple adjacent spinal implants within the same intervertebral space or within the same vertebra, e.g. comprising two adjacent spinal implants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0004—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof bioabsorbable
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0025—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0025—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2220/0033—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements made by longitudinally pushing a protrusion into a complementary-shaped recess, e.g. held by friction fit
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0008—Rounded shapes, e.g. with rounded corners elliptical or oval
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0015—Kidney-shaped, e.g. bean-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0017—Angular shapes
- A61F2230/0019—Angular shapes rectangular
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0017—Angular shapes
- A61F2230/0026—Angular shapes trapezoidal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0065—Three-dimensional shapes toroidal, e.g. ring-shaped, doughnut-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0069—Three-dimensional shapes cylindrical
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0018—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in elasticity, stiffness or compressibility
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0032—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in radiographic density
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/0096—Markers and sensors for detecting a position or changes of a position of an implant, e.g. RF sensors, ultrasound markers
- A61F2250/0098—Markers and sensors for detecting a position or changes of a position of an implant, e.g. RF sensors, ultrasound markers radio-opaque, e.g. radio-opaque markers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00011—Metals or alloys
- A61F2310/00017—Iron- or Fe-based alloys, e.g. stainless steel
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00011—Metals or alloys
- A61F2310/00023—Titanium or titanium-based alloys, e.g. Ti-Ni alloys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00011—Metals or alloys
- A61F2310/00029—Cobalt-based alloys, e.g. Co-Cr alloys or Vitallium
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00011—Metals or alloys
- A61F2310/00035—Other metals or alloys
- A61F2310/00131—Tantalum or Ta-based alloys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00161—Carbon; Graphite
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00179—Ceramics or ceramic-like structures
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00341—Coral, e.g. aragonite, porite
Abstract
一种由至少两种不同材料构成的植入体。该植入体可包括具有各种射线可透过性和机械性能的材料。这种混合植入体能够在植入体设置中,包括用于脊柱关节融合术的设置中提供受控的放射成像视图和优化的结构性能。
Description
技术领域
本发明一般涉及医疗植入体及植入方法领域,更具体地涉及适合置于跨过两个相邻椎体之间的盘间隙高度形成的植入空间内以改善该间隙处的疾患、功能障碍或变性的椎体间脊柱植入体,以及任何相关的方法。脊柱植入体可由多种植入材料构成,这些材料具有不同的放射成像透过程度。这些材料可包括骨,并且这些材料可以是重吸收性或非重吸收性的。一些实施方式的植入体被配置成能够放射成像观察操作放置和最终的骨治愈。
背景技术
用于放置到脊柱相邻椎体间的椎间隙内的植入体可具有多种形状和尺寸。这些植入体通常整个地由一种材料构成,但特定植入体之间所用的材料类型可以不同。适用于人体脊柱外科手术的植入体包括完全由金属构成的植入体,例如钛或不锈钢,或由可透射射线的合成材料如碳-碳复合材料或聚-醚-醚-酮(PEEK)构成的植入体。植入体的结构可被设计成,通过允许骨组织穿过和围绕植入体生长以促进相邻椎体间的融合。通过放射成像不透性可实现植入体在椎体间的最佳操作放置。然而,相对射线可透过性植入体材料有利于椎间隙中骨生长与融合的术后评价。虽然这些植入体可包含标记物或射线不透性标记物,它们在结构上并未得益于射线不透性材料。在一些构型中,金属(其中一些是放射图像上不透明的)在植入期间提供了较大的强度和抵冲击性。金属植入体可实现结构元件较小的壁厚度,从而为骨移植物和植入体中的其它试剂提供更大的空间。
由于植入体中希望利用射线可透过性和射线不透性的材料,因而需要具有不同放射成像外观性能的不同结构性材料构成的改良植入体。对于一些植入体来说,希望优化机械性能,同时实现充分的骨填充和骨穿透生长。在一些实施方式中可应用这些特征,从而经放射成像来确定骨植入体的相互作用以及骨生长进入和围绕植入体生长。
发明内容
本发明的实施方式可包括人造椎间脊柱融合植入体,所述植入体由各种射线可透过性和机械性能的结构性材料构成。提供植入体,用于至少部分地插入跨过人体脊柱相邻椎体间盘间隙高度形成的植入空间内。一些实施方式的植入体由至少两个放射成像上不同的成像材料构成:即射线可透过性部分和射线不透性部分。一些实施方式的射线不透性材料被设置成面向椎骨终板,最低程度地妨碍前到后和/或横向侧方向穿过植入体的放射成像观察。植入体的实施方式可包括上部分和下部分,它们被置于椎间隙内以接触和支撑相邻椎体。植入体的上下部分可包括至少一个相互连通的开口,它们被配置成能够容纳骨生长促进材料和/或骨移植物,从而允许骨组织穿过植入体从椎体生长到椎体。本发明的实施方式包括含有至少两种不同材料的人造椎体间脊柱植入体,用于至少部分地插入跨过脊柱相邻椎体间盘间隙高度形成的植入空间内。植入体实施方式可采用在植入体的设计中具有结构性作用的材料,植入体引导端的至少一部分的高度降低以便于所述植入体插入到相邻椎体之间。植入体的最大长度小于且近似于椎体的后到前或右到左长度。一些实施方式还包括至少在上下部分的外表面上形成的用于与相邻椎体啮合的骨啮合面,例如一个或多个凸起、棘齿、销钉、粗糙面或滚花。植入体的实施方式可与骨生长或骨治愈促进性材料联用,这些材料包括但不限于:骨、骨衍生产品、脱矿骨基质、矿化蛋白、骨化蛋白、骨成形细胞分化物质、骨形态发生蛋白、羟基磷灰石以及导致骨生成的基因治疗物质。植入体的实施方式也可与用于治疗感染、肿瘤或其它病理学过程的治疗性物质联用。在本发明的一些实施方式中,一种组成材料相对,或绝对射线可透过。在本发明的一些实施方式中,一种组成材料是射线不透性的。植入体的一种组成材料是至少部分地可重吸收的。在一些实施方式中,至少一部分植入体经处理以促进植入体与相邻椎体间的骨向内生长。植入体的实施方式可与至少一种脊柱固定植入体联用。植入体的实施方式可包括内部空腔和至少一个用于与插入装置附连或相互作用的区域,用于将植入体手术放置到椎间隙中或从中取出。一些实施方式的植入体的上下表面可包括多个开口。植入体的实施方式可被设计成能够在第二植入体附近插入相邻椎体的盘间隙内,所述第二植入体可为是相同或不同形状。至少一个开口可位于植入体实施方式的前端和尾端之间。植入体实施方式的上下部分或表面可至少部分地相互间大致平行,或者可被构造成相互间成一定角度以适应相邻椎体相互间的角度关系。
本发明的另一个实施方式是用于促进相邻椎体间融合的椎间植入体。该植入物可包括至少部分地由第一材料构成的第一主体,第一主体具有下方横向延伸元件,联接于下方横向延伸元件并从其向上延伸的支持件,及联接于支持件的上方横向延伸元件。所述植入体还包括至少部分地由第二材料构成第二主体,第二主体被构造成至少部分地匹配在下方横向延伸元件和上方横向延伸元件之间。
本发明的又一个实施方式是用于促进相邻椎体间融合的椎间植入体。该植入体可包括用于啮合相邻椎体的相对终板的第一和第二放射不透性板,第一和第二板被构造成在其间形成空间,以及在所述空间的相对横向侧位于所述第一和第二板之间的第一和第二放射可透过性模块。第一和第二板之间的空间内可形成内部空腔,所述内部空腔至少在两侧上被第一和第二射线可透过性模块部分地包封。
本发明的又一个实施方式是一种具有横向径、前-后径、下-上垂直径的椎间植入体。该植入体可包括:下方横向延伸元件;上方横向延伸元件;大致射线可透过的主体,它被构造成至少部分地匹配在下方横向延伸元件与上方横向延伸元件之间。植入体还包括联接于下方横向延伸元件和上方横向延伸元件并在其间延伸的两个或多个支持件,从前侧、后侧、横向侧中至少一个放射成像角度观察,所述两个或多个支持件的相对排列指示了植入体围绕垂直轴线的转动位置。
附图说明
图1是腰椎中两个相邻椎体的侧视图,跨过脊柱盘间隙高度形成植入空间。
图2是腰椎中椎体的俯视图,通过后侧路径形成植入空间。
图3是图2所示植入空间的立体图。
图4是通过前侧路径形成植入空间的立体图。
图5是腰椎中椎体的俯视图,一个实施方式的植入体位于图2所示的植入空间中。
图6是两个相邻椎体的侧视图,图5所示植入体通过后侧路径位于图2所示的植入空间中。
图7是两个相邻椎体的侧视图,植入体通过前侧路径位于图2所示的植入空间中。
图8是图5所示植入体的俯视图。
图9是图5所示植入体的后视立体图。
图10是图5所示植入体的侧视图。
图11是图5所示植入体的后视图。
图12是用于图2所示植入空间中的另一个实施方式的植入体的后视立体图。
图13是图12所示植入体的后视图。
图14是图12所示植入体的侧视图。
图15是适用于前侧放置到颈部或腰部椎体间盘间隙内的一个实施方式的植入体的后视图。
图16是图15所示植入体的俯视图。
图17是图15所示植入体的侧视图。
具体实施方式
下面的描述是为了示意而不是限制性的,根据这些内容可进行许多变化,这些变化形式也包括在本发明的范围内。现在具体参考本发明的实施方式,这些实施方式的例子在附图中显示。可能的话,所有附图中相同的附图标记用于表示相同或类似的部件。
图1-3显示了跨过腰椎中椎体V之间的椎间盘D的高度形成的植入空间100。在其它实施方式中,椎体可以是颈椎或胸椎的椎体。应理解,存在许多方法,并且可采用任何适合该目的的方法和器械来制备所需的植入空间并清除椎间盘和软组织,从而适于接纳本发明的植入体。还应理解,植入空间的制备通常在植入体设置之前保留了残余的盘物质D。
图3显示了通过部分清除椎体V附近的盘和软组织制备得到的植入空间100。图3中的制备为后侧腰部外科手术路径,显示了从后侧进入盘间隙内的开口O。开口O也可以是经椎间孔或倾斜的外科手术路径制备的。还显示了椎弓根的残余部分P。
图4显示了通过部分清除椎体V附近的盘和软组织制备得到的植入空间100。图4中的制备为前侧外科手术路径,显示了从前侧进入盘间隙内的进口E。该图示可反映颈椎、胸椎或腰椎的椎间隙制备。
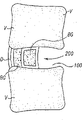
图5显示了根据本发明的实施方式、位于植入空间100内的单侧植入体200。显示骨移植物材料BG在单侧植入体200的前侧,并且在单侧植入体200的中央空穴210中。
图6显示了位于植入空间100内的单侧植入体200。显示骨移植物材料BG在单侧植入体200的前侧但在残余盘物质D的后侧,并且在单侧植入体200的中央空穴210中。
图7显示了位于植入空间100内的单侧植入体400。显示骨移植物材料BG在单侧植入体400的空穴480内。
图8显示了单侧植入体200,它具有前侧202和后侧204。显示了中央空穴210。横向支持结构220、220’从植入体的前侧202延伸到后侧204。在单侧植入体200的横向侧显示了射线透性模块240、240’,各自具有中央空穴242、242’。
图9显示了图8所述的单侧植入体200。后侧立体图显示了中央空腔210、射线可透过性模块240、240’和后支撑柱222、222’和从植入体的下侧260延伸到上侧264的支撑柱222、222’。
图10从侧视图的角度显示了图8所述的单侧植入体200。显示射线可透过性模块240位于植入体的上侧264和下侧260之间。显示了在上侧264和下侧260之间的后支撑柱222和前支撑柱223。在侧投影中,可注意到植入体的前侧202和后侧204。
图11显示了没有射线可透过性模块240、240’的图8和9所述植入体的后视图,以显示放射成像外观。
由于该实施方式中所用材料的射线不透性,放射图像上仅显示了在植入体的下侧260和上侧264间延伸的后支撑柱222、222’。当从后侧直接通过放射成像观察单侧植入体200时,前支撑柱223、223’隐藏在后支撑柱222、222’的后面。
图12显示了本发明另一个实施方式的中央支撑植入体300的后侧立体图。可注意到中央容积310、射线可透过性横向模块340、340’以及前支持结构324和后支持结构322。
图13显示了没有射线可透过性横向模块340、340’的图12所述植入体的后视图,以显示放射成像外观。由于该实施方式所用材料的射线不透性,放射图像上仅显示了在植入体的下侧360与上侧364间延伸的后支持结构322,后支持结构322在此图中与图12中可见的前支持结构324重叠。
图14显示了图12所示中央支撑植入体300的侧视图。显示射线可透过性横向模块340位于植入体的上侧364与下侧360之间。该侧投影中,示出了植入体的前支持结构324和后支持结构322。
图15显示了前侧植入体400。在一些实施方式中,前侧植入体400可通过前侧外科手术路径设置。然而,前侧植入体400也可通过其它外科手术路径设置,例如但不限于前侧-倾斜路径或横向路径。显示由射线可透过性材料构成的大的中央支柱410横跨植入体。上缘边420和下缘边422附连于中央支柱410,并通过支持结构440、442、444、446相互连接。示出了穿过植入体侧面的开口450、452、454、456。这些开口允许骨组织生长穿过和进入前侧植入体400,但本发明并不限于此。
图16显示了图15所述前侧植入体400的俯视图。可注意到大的中央支柱410。显示前侧植入体400内在支柱410的两侧具有两个空穴480、480’。这些空穴允许骨组织穿过和进入前侧植入体400,但本发明并不限于此。
图17显示了图15和16所示前侧植入体400的侧视图。显示了上缘边420和下缘边422,以及中央支柱410的侧视图。由于中央支柱410的射线可透过性,在放射成像视图上仅示出了上缘边420和下缘边422以及射线不透性支持结构440、442。侧视图中,由于支持结构440、442而使图15所示其它两个支持结构444、446被遮挡。
而且,上缘边420和下缘边422间的角度有利于前侧植入体400插入到两个相邻椎体之间和控制矢状面椎间对准。
虽然所述植入体主要用于脊柱融合,应理解它们被经改良或配置成内部能够接纳融合促进性物质和/或材料,例如但不限于松质骨、骨衍生产品、化疗剂、抗微生物剂等。在一些实施方式中,植入体由以下材料构成,例如但不限于:钛及其合金、ASTM材料、钴铬、钽、陶瓷、聚-醚-醚-酮(PEEK)、各种塑料、塑料复合材料、碳纤维复合材料、珊瑚,并可包括至少部分地可生物重吸收的人工材料。植入体所用结构性材料的放射成像外观具有各种性质,从而实现植入体设置、植入体-骨界面和/或骨生长进入和贯穿生长的最佳观察。
虽然上述说明揭示了植入体上下表面的各种关系,平行或不平行,但应注意表面间的轻度倾斜也是可能的。这样,植入脊柱后,这些植入体允许相邻椎体相互间以倾斜关系定位,从而恢复脊柱的自然曲率,例如脊柱前凸等。还应注意,植入体的形状也可以显著变化,包括但不限于:肾形、圆形、楔形、圆柱形、梯形、矩形、长方形和椭圆形。
外表面可具有螺纹或特定的不均匀性,以便于插入或锚定到周围组织或骨中。在本发明任一实施方式中,植入体可包括、由以下材料构成、处理、涂覆、填充或联用适合植入人体脊柱的人造或天然产生的材料和/或物质、或者也可具有空腔或开口以容纳这些材料和/或物质。这些材料和/或物质包括以下任一来源:骨发生、骨生长促进性材料、骨、骨衍生物质或产品、脱矿骨基质、矿化蛋白、骨化蛋白、骨形态发生蛋白、羟基磷灰石、用于生成骨的基因编码,骨包括但不限于皮质骨、抗生素、癌症治疗物质、感染治疗物质或其它疾病治疗物质。这些植入体至少部分地包括体内可生物重吸收和/或可重吸收的材料。本发明的植入体可由多孔材料形成,或者由固有参与相邻椎骨间骨胳生长的材料构成。至少一部分植入体经处理,以促进植入体和相邻椎体间的骨向内生长。
本发明植入体可与脊柱固定装置联用,所述固定装置包括可插入任意脊柱部分的任何装置而不论材料类型,例如但不限于椎体间脊柱植入体、结构性骨移植物、网状物、保持架、间隔物、销钉、骨螺钉、板、杆、合成材料的系绳或线、或其它脊柱固定装置。虽然参考具体实施方式描述了本发明,但本领域普通技术人员应理解,本发明可进行各种改进而不背离其精神和范围。在这里预期且要求在本发明精神内的所有这些改变和改进。
Claims (27)
1.一种用于促进相邻椎体间融合的椎间植入体,其包括:
至少部分地由第一材料构成的第一主体,所述第一主体包括:
下方横向延伸元件,
联接于所述下方横向延伸元件并从其向上延伸的支持件,和
联接于支持件的上方横向延伸元件;和
至少部分地由第二材料构成的第二主体,所述第二主体被构造成至少部分地配合在下方横向延伸元件与上方横向延伸元件之间。
2.如权利要求1所述的椎间植入体,其特征在于,所述第一材料是生物相容性金属。
3.如权利要求1所述的椎间植入体,其特征在于,所述第一材料是钛。
4.如权利要求1所述的椎间植入体,其特征在于,所述第一材料是放射成像可检测的。
5.如权利要求1所述的椎间植入体,其特征在于,所述第一材料是不透射线的。
6.如权利要求1所述的椎间植入体,其特征在于,所述下方横向延伸元件是被构造成至少部分地接触下椎体的板。
7.如权利要求1所述的椎间植入体,其特征在于,所述下方横向延伸元件包括一个或多个指向下椎体的凸起、棘齿、销钉、粗糙面和滚花。
8.如权利要求1所述的椎间植入体,其特征在于,所述支持件包括能够在下方横向延伸元件与上方横向延伸元件之间提供结构支撑的柱。
9.如权利要求1所述的椎间植入体,其特征在于,所述上方横向延伸元件是被构造成至少部分地接触上椎体的板。
10.如权利要求1所述的椎间植入体,其特征在于,所述上方横向延伸元件包括一个或多个指向上椎体的凸起、棘齿、销钉、粗糙面和滚花。
11.如权利要求1所述的椎间植入体,其特征在于,所述第二材料是生物相容性聚合物。
12.如权利要求1所述的椎间植入体,其特征在于,所述第二材料是PEEK。
13.如权利要求1所述的椎间植入体,其特征在于,所述第二材料是放射成像可检测的。
14.如权利要求1所述的椎间植入体,其特征在于,所述第二材料是射线可透过的。
15.如权利要求1所述的椎间植入体,其特征在于,所述第二材料的放射成像可检测性低于所述第一材料。
16.如权利要求1所述的椎间植入体,所述植入体还包括在下方横向延伸元件与上方横向延伸元件之间的骨生长促进物质。
17.一种用于促进两个相邻椎体间融合的椎间植入体,其包括:
用于啮合相邻椎体的相对终板的第一和第二射线不透性板,所述第一和第二板被构造成在其间形成空间;
在所述空间的相对横向侧的位于第一和第二板之间的第一和第二射线可透过性模块;
其中,在第一和第二板之间的空间内形成内部空腔,所述内部空腔至少在两侧上被第一和第二射线可透过性模块部分地包封。
18.如权利要求17所述的椎间植入体,其特征在于,所述第一和第二射线不透性板至少部分地由钛构成。
19.如权利要求17所述的椎间植入体,其特征在于,所述第一和第二射线可透过性模块至少部分地由PEEK构成。
20.如权利要求17所述的椎间植入体,其特征在于,所述第一和第二射线可透过性模块中至少一个包括穿过射线可透过性模块的孔。
21.如权利要求20所述的椎间植入体,所述植入体还包括穿过所述射线可透过性模块的孔之一或二者的骨生长促进物质。
22.如权利要求17所述的椎间植入体,其特征在于,当植入所述植入体时,所述内部空腔在相邻椎体间延伸。
23.如权利要求17所述的椎间植入体,所述植入体还包括在所述内部空腔内的骨生长促进物质。
24.一种具有横向径、前-后径、下-上垂直径的椎间植入体,所述植入体用于设置到相邻椎体之间,其包括:
下方横向延伸元件;
上方横向延伸元件;
大致射线可透过的主体,它被构造成至少部分地配合在下方横向延伸元件与上方横向延伸元件之间;
联接于下方横向延伸元件和上方横向延伸元件并在其间延伸的两个或多个支持件;
其中,从前侧、后侧、横向侧中至少一个放射成像观察,所述两个或多个支持件的相对排列指示了植入体围绕垂直轴线的转动位置。
25.如权利要求24所述的椎间植入体,其特征在于,植入体后侧附近的第一支持件位于植入体前侧附近的第二支持件的正后方,使得当从植入体正后方放射成像观察时,仅看到第一支持件。
26.如权利要求24所述的椎间植入体,其特征在于,植入体后侧附近的第一支持件和第三支持件分别位于植入体前侧附近的第二支持件和第四支持件的正后方,使得当从植入体正后方放射成像观察时,仅看到第一和第三支持件。
27.如权利要求24所述的椎间植入体,其特征在于,植入体第一横向侧附近的第一支持件和第二支持件分别位于相对于第一横向侧的第二横向侧附近的第三支持件和第四支持件的横向侧,使得当从第一横向侧放射成像观察时,仅看到第一和第二支持件。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US72055505P | 2005-09-26 | 2005-09-26 | |
| US60/720,555 | 2005-09-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN101272750A true CN101272750A (zh) | 2008-09-24 |
Family
ID=37668060
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA2006800352540A Pending CN101272749A (zh) | 2005-09-26 | 2006-09-26 | 前侧混合植入体 |
| CNA200680035256XA Pending CN101296669A (zh) | 2005-09-26 | 2006-09-26 | 经椎间孔的混合植入物 |
| CNA2006800352555A Pending CN101272750A (zh) | 2005-09-26 | 2006-09-26 | 混合脊柱融合椎间植入体 |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA2006800352540A Pending CN101272749A (zh) | 2005-09-26 | 2006-09-26 | 前侧混合植入体 |
| CNA200680035256XA Pending CN101296669A (zh) | 2005-09-26 | 2006-09-26 | 经椎间孔的混合植入物 |
Country Status (5)
| Country | Link |
|---|---|
| US (3) | US7998212B2 (zh) |
| EP (1) | EP1951164A1 (zh) |
| JP (1) | JP2009509590A (zh) |
| CN (3) | CN101272749A (zh) |
| WO (1) | WO2007038545A1 (zh) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105997310A (zh) * | 2016-05-06 | 2016-10-12 | 北京市春立正达医疗器械股份有限公司 | 骨小梁融合器 |
Families Citing this family (120)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4398975B2 (ja) | 2003-01-31 | 2010-01-13 | スパイナルモーション, インコーポレイテッド | 脊髄中線指示器 |
| WO2004066884A1 (en) | 2003-01-31 | 2004-08-12 | Spinalmotion, Inc. | Intervertebral prosthesis placement instrument |
| WO2004073563A2 (en) | 2003-02-14 | 2004-09-02 | Depuy Spine, Inc. | In-situ formed intervertebral fusion device |
| US10052211B2 (en) | 2003-05-27 | 2018-08-21 | Simplify Medical Pty Ltd. | Prosthetic disc for intervertebral insertion |
| US7575599B2 (en) | 2004-07-30 | 2009-08-18 | Spinalmotion, Inc. | Intervertebral prosthetic disc with metallic core |
| EP2161008B1 (en) | 2003-05-27 | 2014-12-24 | Simplify Medical, Inc. | Method for assembling a prosthetic disc for intervertebral insertion |
| US20040267367A1 (en) | 2003-06-30 | 2004-12-30 | Depuy Acromed, Inc | Intervertebral implant with conformable endplate |
| US8636802B2 (en) | 2004-03-06 | 2014-01-28 | DePuy Synthes Products, LLC | Dynamized interspinal implant |
| US7585326B2 (en) | 2004-08-06 | 2009-09-08 | Spinalmotion, Inc. | Methods and apparatus for intervertebral disc prosthesis insertion |
| US20180228621A1 (en) | 2004-08-09 | 2018-08-16 | Mark A. Reiley | Apparatus, systems, and methods for the fixation or fusion of bone |
| US8083797B2 (en) | 2005-02-04 | 2011-12-27 | Spinalmotion, Inc. | Intervertebral prosthetic disc with shock absorption |
| US8758443B2 (en) | 2005-05-06 | 2014-06-24 | Titan Spine, Llc | Implants with integration surfaces having regular repeating surface patterns |
| US9168147B2 (en) | 2005-05-06 | 2015-10-27 | Titan Spine, Llc | Self-deploying locking screw retention device |
| US8585765B2 (en) | 2005-05-06 | 2013-11-19 | Titan Spine, Llc | Endplate-preserving spinal implant having a raised expulsion-resistant edge |
| US8617248B2 (en) | 2005-05-06 | 2013-12-31 | Titan Spine, Llc | Spinal implant having variable ratios of the integration surface area to the axial passage area |
| US11096796B2 (en) | 2005-05-06 | 2021-08-24 | Titan Spine, Llc | Interbody spinal implant having a roughened surface topography on one or more internal surfaces |
| US8403991B2 (en) | 2005-05-06 | 2013-03-26 | Titan Spine Llc | Implant with critical ratio of load bearing surface area to central opening area |
| US8814939B2 (en) | 2005-05-06 | 2014-08-26 | Titan Spine, Llc | Implants having three distinct surfaces |
| US8585767B2 (en) | 2005-05-06 | 2013-11-19 | Titan Spine, Llc | Endplate-preserving spinal implant with an integration plate having durable connectors |
| US8591590B2 (en) | 2005-05-06 | 2013-11-26 | Titan Spine, Llc | Spinal implant having a transverse aperture |
| US8435302B2 (en) | 2005-05-06 | 2013-05-07 | Titan Spine, Llc | Instruments and interbody spinal implants enhancing disc space distraction |
| US8551176B2 (en) | 2005-05-06 | 2013-10-08 | Titan Spine, Llc | Spinal implant having a passage for enhancing contact between bone graft material and cortical endplate bone |
| US20120312779A1 (en) | 2005-05-06 | 2012-12-13 | Titian Spine, LLC | Methods for manufacturing implants having integration surfaces |
| US8562684B2 (en) | 2005-05-06 | 2013-10-22 | Titan Spine, Llc | Endplate-preserving spinal implant with an integration plate having a roughened surface topography |
| US8992622B2 (en) | 2005-05-06 | 2015-03-31 | Titan Spine, Llc | Interbody spinal implant having a roughened surface topography |
| US8480749B2 (en) | 2005-05-06 | 2013-07-09 | Titan Spine, Llc | Friction fit and vertebral endplate-preserving spinal implant |
| US8562685B2 (en) | 2005-05-06 | 2013-10-22 | Titan Spine, Llc | Spinal implant and integration plate for optimizing vertebral endplate contact load-bearing edges |
| US8585766B2 (en) | 2005-05-06 | 2013-11-19 | Titan Spine, Llc | Endplate-preserving spinal implant with an integration plate having durable connectors |
| US8758442B2 (en) | 2005-05-06 | 2014-06-24 | Titan Spine, Llc | Composite implants having integration surfaces composed of a regular repeating pattern |
| US9125756B2 (en) | 2005-05-06 | 2015-09-08 | Titan Spine, Llc | Processes for producing regular repeating patterns on surfaces of interbody devices |
| US8545568B2 (en) | 2005-05-06 | 2013-10-01 | Titan Spine, Llc | Method of using instruments and interbody spinal implants to enhance distraction |
| US8262737B2 (en) * | 2005-05-06 | 2012-09-11 | Titan Spine, Llc | Composite interbody spinal implant having openings of predetermined size and shape |
| US8409290B2 (en) * | 2006-03-08 | 2013-04-02 | Seaspine, Inc. | Interbody device for spinal applications |
| WO2007121320A2 (en) * | 2006-04-12 | 2007-10-25 | Spinalmotion, Inc. | Posterior spinal device and method |
| EP1849437B1 (en) * | 2006-04-28 | 2009-09-30 | Concept Matrix, LLC | Dual composition vertebral fixation device |
| US20080077247A1 (en) * | 2006-09-22 | 2008-03-27 | Alphatec Spine, Inc. | Spinal spacer |
| WO2008070863A2 (en) | 2006-12-07 | 2008-06-12 | Interventional Spine, Inc. | Intervertebral implant |
| US8172905B2 (en) * | 2007-04-27 | 2012-05-08 | Atlas Spine, Inc. | Spinal implant |
| US8083799B2 (en) * | 2007-04-27 | 2011-12-27 | Atlas Spine, Inc. | Spinal implant |
| US8900307B2 (en) | 2007-06-26 | 2014-12-02 | DePuy Synthes Products, LLC | Highly lordosed fusion cage |
| US20090043391A1 (en) | 2007-08-09 | 2009-02-12 | Spinalmotion, Inc. | Customized Intervertebral Prosthetic Disc with Shock Absorption |
| EP2209444A4 (en) | 2007-10-22 | 2013-03-27 | Spinalmotion Inc | DYNAMIC SPACER DEVICE AND METHOD FOR COVERING A SPACE FORMED DURING THE REMOVAL OF AN INTERVERTEBRAL DISC |
| JP5441922B2 (ja) | 2008-01-17 | 2014-03-12 | ジンテス ゲゼルシャフト ミット ベシュレンクテル ハフツング | 膨張可能な椎間インプラント及び関連するその製造方法 |
| EP2262449B1 (en) | 2008-04-05 | 2020-03-11 | Synthes GmbH | Expandable intervertebral implant |
| US9034038B2 (en) | 2008-04-11 | 2015-05-19 | Spinalmotion, Inc. | Motion limiting insert for an artificial intervertebral disc |
| EP2278941A1 (en) | 2008-05-05 | 2011-02-02 | Spinalmotion Inc. | Polyaryletherketone artificial intervertebral disc |
| US9220603B2 (en) | 2008-07-02 | 2015-12-29 | Simplify Medical, Inc. | Limited motion prosthetic intervertebral disc |
| EP2299944A4 (en) | 2008-07-17 | 2013-07-31 | Spinalmotion Inc | SYSTEM FOR INSTALLING ARTIFICIAL INTERVERTEBRAL DISCS |
| WO2010009153A1 (en) | 2008-07-18 | 2010-01-21 | Spinalmotion, Inc. | Posterior prosthetic intervertebral disc |
| US8545566B2 (en) * | 2008-10-13 | 2013-10-01 | Globus Medical, Inc. | Articulating spacer |
| US20100158209A1 (en) * | 2008-12-22 | 2010-06-24 | General Instrument Corporation | Access to Network Based on Automatic Speech-Recognition |
| US9220547B2 (en) | 2009-03-27 | 2015-12-29 | Spinal Elements, Inc. | Flanged interbody fusion device |
| US9526620B2 (en) | 2009-03-30 | 2016-12-27 | DePuy Synthes Products, Inc. | Zero profile spinal fusion cage |
| EP2419053B1 (en) * | 2009-04-15 | 2015-04-08 | Synthes GmbH | Trial implant assembly |
| US9216023B2 (en) | 2009-05-08 | 2015-12-22 | DePuy Synthes Products, Inc. | Expandable bone implant |
| BRPI1012942B8 (pt) | 2009-07-09 | 2021-06-22 | R Tree Innovations Llc | sistema para inserção de um dispositivo intercorporal em um espaço de disco entre vértebras adjacentes |
| US8491658B1 (en) | 2009-07-12 | 2013-07-23 | Mohammad Etminan | Interbody fusion implant and related methods |
| US9247970B2 (en) * | 2009-08-19 | 2016-02-02 | DePuy Synthes Products, Inc. | Method and apparatus for augmenting bone |
| US8480748B2 (en) * | 2010-10-07 | 2013-07-09 | Nicholas Poulos | Lordotic expandable interbody implant and method |
| US9393129B2 (en) | 2009-12-10 | 2016-07-19 | DePuy Synthes Products, Inc. | Bellows-like expandable interbody fusion cage |
| US8945226B2 (en) * | 2009-12-31 | 2015-02-03 | Rhausler | Vertebral spacer |
| US9408718B2 (en) | 2009-12-31 | 2016-08-09 | Rhausler, Inc. | Inter-vertebral implant with angled plate |
| US8845733B2 (en) | 2010-06-24 | 2014-09-30 | DePuy Synthes Products, LLC | Lateral spondylolisthesis reduction cage |
| US8979860B2 (en) | 2010-06-24 | 2015-03-17 | DePuy Synthes Products. LLC | Enhanced cage insertion device |
| TW201215379A (en) | 2010-06-29 | 2012-04-16 | Synthes Gmbh | Distractible intervertebral implant |
| US9402736B2 (en) * | 2010-07-12 | 2016-08-02 | Alphatec Spine, Inc. | Interbody fusion implant and related methods |
| US9402732B2 (en) | 2010-10-11 | 2016-08-02 | DePuy Synthes Products, Inc. | Expandable interspinous process spacer implant |
| EP2691051B1 (en) * | 2011-03-30 | 2017-09-13 | Trinity Orthopedics, LLC | Articulating interbody cage |
| JP5985617B2 (ja) * | 2011-05-25 | 2016-09-06 | シンセス・ゲーエムベーハーSynthes GmbH | 放射線不透過性マーカーを有する照準装置 |
| AT12506U1 (de) * | 2011-05-27 | 2012-06-15 | Sabitzer Ronald J Dr | Implantat |
| US8992619B2 (en) | 2011-11-01 | 2015-03-31 | Titan Spine, Llc | Microstructured implant surfaces |
| BR112014010427A2 (pt) * | 2011-11-01 | 2017-04-18 | Amedica Corp | implantes com elemento de inserção conectável e sistemas e métodos relacionados |
| US8795167B2 (en) | 2011-11-15 | 2014-08-05 | Baxano Surgical, Inc. | Spinal therapy lateral approach access instruments |
| CN102512230B (zh) * | 2011-12-09 | 2013-06-05 | 北京奥精医药科技有限公司 | 一种内衬加筋的脊柱融合器 |
| US10363140B2 (en) | 2012-03-09 | 2019-07-30 | Si-Bone Inc. | Systems, device, and methods for joint fusion |
| CN104334102A (zh) | 2012-03-09 | 2015-02-04 | 西-博恩公司 | 整合种植体 |
| EP2827806B1 (en) | 2012-03-20 | 2020-06-24 | Titan Spine, Inc. | Process of fabricating bioactive spinal implant endplates |
| EP2846705B1 (en) | 2012-05-04 | 2018-12-26 | SI-Bone, Inc. | Fenestrated implant |
| US9510955B2 (en) | 2012-05-18 | 2016-12-06 | Trinity Orthopedics, Llc | Articulating interbody cage and methods thereof |
| US9585764B2 (en) * | 2012-07-26 | 2017-03-07 | Warsaw Orthopedic, Inc. | Bone implant device |
| EP2716261A1 (en) | 2012-10-02 | 2014-04-09 | Titan Spine, LLC | Implants with self-deploying anchors |
| US9498349B2 (en) | 2012-10-09 | 2016-11-22 | Titan Spine, Llc | Expandable spinal implant with expansion wedge and anchor |
| US9522070B2 (en) | 2013-03-07 | 2016-12-20 | Interventional Spine, Inc. | Intervertebral implant |
| US9238319B2 (en) | 2013-03-14 | 2016-01-19 | DePuy Synthes Products, Inc. | Hybrid intervertebral disc spacer device and method of manufacturing the same |
| US9936983B2 (en) | 2013-03-15 | 2018-04-10 | Si-Bone Inc. | Implants for spinal fixation or fusion |
| US11147688B2 (en) | 2013-10-15 | 2021-10-19 | Si-Bone Inc. | Implant placement |
| US9615935B2 (en) | 2014-01-30 | 2017-04-11 | Titan Spine, Llc | Thermally activated shape memory spring assemblies for implant expansion |
| US9566169B2 (en) | 2014-03-13 | 2017-02-14 | DePuy Synthes Products, Inc. | ACIS allograft designs |
| US10166033B2 (en) | 2014-09-18 | 2019-01-01 | Si-Bone Inc. | Implants for bone fixation or fusion |
| US9662157B2 (en) * | 2014-09-18 | 2017-05-30 | Si-Bone Inc. | Matrix implant |
| US9918851B2 (en) * | 2014-10-22 | 2018-03-20 | Stryker European Holdings I, Llc | Spinal fusion implant |
| CN105686895A (zh) * | 2014-12-16 | 2016-06-22 | 孙建忠 | 一种生物解剖型颈前路植骨骨块 |
| US11426290B2 (en) | 2015-03-06 | 2022-08-30 | DePuy Synthes Products, Inc. | Expandable intervertebral implant, system, kit and method |
| WO2018002711A2 (en) | 2016-06-28 | 2018-01-04 | Eit Emerging Implant Technologies Gmbh | Expandable, angularly adjustable intervertebral cages |
| WO2018002715A2 (en) | 2016-06-28 | 2018-01-04 | Eit Emerging Implant Technologies Gmbh | Expandable and angularly adjustable articulating intervertebral cages |
| EP3515368A1 (en) * | 2016-09-20 | 2019-07-31 | DePuy Synthes Products, Inc. | Intervertebral implant |
| US10888433B2 (en) | 2016-12-14 | 2021-01-12 | DePuy Synthes Products, Inc. | Intervertebral implant inserter and related methods |
| US10675159B2 (en) * | 2017-02-08 | 2020-06-09 | Osseus Fusion Systems, Llc | Composite interbody system |
| US10398563B2 (en) | 2017-05-08 | 2019-09-03 | Medos International Sarl | Expandable cage |
| US10624760B2 (en) | 2017-05-22 | 2020-04-21 | Warsaw Orthopedic, Inc. | Spinal implant system and method |
| US11344424B2 (en) | 2017-06-14 | 2022-05-31 | Medos International Sarl | Expandable intervertebral implant and related methods |
| US10940016B2 (en) | 2017-07-05 | 2021-03-09 | Medos International Sarl | Expandable intervertebral fusion cage |
| WO2019067584A1 (en) | 2017-09-26 | 2019-04-04 | Si-Bone Inc. | SYSTEMS AND METHODS FOR DECORTICATING SACROILITIC JOINT |
| US11766339B1 (en) | 2017-10-24 | 2023-09-26 | Omnia Medical, LLC | Multi-material multi-component spinal implant |
| US10918497B1 (en) | 2017-10-24 | 2021-02-16 | Omnia Medical, LLC | Multi-material multi-component spinal implant |
| US10973658B2 (en) | 2017-11-27 | 2021-04-13 | Titan Spine, Inc. | Rotating implant and associated instrumentation |
| US11135070B2 (en) | 2018-02-14 | 2021-10-05 | Titan Spine, Inc. | Modular adjustable corpectomy cage |
| US10736756B2 (en) * | 2018-03-30 | 2020-08-11 | Warsaw Orthopedic, Inc. | Radiolucent trial |
| CN108888389A (zh) * | 2018-09-14 | 2018-11-27 | 北京爱康宜诚医疗器材有限公司 | 关节面融合器 |
| EP3852690A4 (en) | 2018-09-20 | 2022-10-26 | Spinal Elements, Inc. | SPINAL IMPLANT DEVICE |
| US11446156B2 (en) | 2018-10-25 | 2022-09-20 | Medos International Sarl | Expandable intervertebral implant, inserter instrument, and related methods |
| EP3923829A4 (en) | 2019-02-14 | 2022-12-14 | SI-Bone, Inc. | IMPLANTS FOR SPINE FIXATION AND/OR FUSION |
| US11369419B2 (en) | 2019-02-14 | 2022-06-28 | Si-Bone Inc. | Implants for spinal fixation and or fusion |
| WO2021108590A1 (en) | 2019-11-27 | 2021-06-03 | Si-Bone, Inc. | Bone stabilizing implants and methods of placement across si joints |
| US11426286B2 (en) | 2020-03-06 | 2022-08-30 | Eit Emerging Implant Technologies Gmbh | Expandable intervertebral implant |
| US11166825B1 (en) | 2020-07-01 | 2021-11-09 | Warsaw Orthopedic, Inc. | Spinal implant |
| US11911284B2 (en) | 2020-11-19 | 2024-02-27 | Spinal Elements, Inc. | Curved expandable interbody devices and deployment tools |
| JP2023553120A (ja) | 2020-12-09 | 2023-12-20 | エスアイ-ボーン・インコーポレイテッド | 仙腸関節安定化インプラントおよびインプラント方法 |
| US11850160B2 (en) | 2021-03-26 | 2023-12-26 | Medos International Sarl | Expandable lordotic intervertebral fusion cage |
| US11752009B2 (en) | 2021-04-06 | 2023-09-12 | Medos International Sarl | Expandable intervertebral fusion cage |
Family Cites Families (70)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA1318469C (en) * | 1989-02-15 | 1993-06-01 | Acromed Corporation | Artificial disc |
| US5192327A (en) * | 1991-03-22 | 1993-03-09 | Brantigan John W | Surgical prosthetic implant for vertebrae |
| US5397364A (en) * | 1993-10-12 | 1995-03-14 | Danek Medical, Inc. | Anterior interbody fusion device |
| US6039762A (en) * | 1995-06-07 | 2000-03-21 | Sdgi Holdings, Inc. | Reinforced bone graft substitutes |
| US5782830A (en) * | 1995-10-16 | 1998-07-21 | Sdgi Holdings, Inc. | Implant insertion device |
| US6712819B2 (en) * | 1998-10-20 | 2004-03-30 | St. Francis Medical Technologies, Inc. | Mating insertion instruments for spinal implants and methods of use |
| US6159215A (en) * | 1997-12-19 | 2000-12-12 | Depuy Acromed, Inc. | Insertion instruments and method for delivering a vertebral body spacer |
| AT405367B (de) * | 1998-01-23 | 1999-07-26 | Fuss Franz K Dipl Biomech Dr | Implantat |
| DE19903763B4 (de) * | 1998-01-23 | 2005-03-31 | Aesculap Ag & Co. Kg | Zwischenwirbelimplantat |
| US6224631B1 (en) * | 1998-03-20 | 2001-05-01 | Sulzer Spine-Tech Inc. | Intervertebral implant with reduced contact area and method |
| US6241769B1 (en) * | 1998-05-06 | 2001-06-05 | Cortek, Inc. | Implant for spinal fusion |
| US6090143A (en) | 1998-09-21 | 2000-07-18 | Meriwether; Michael W. | Box cage for intervertebral body fusion |
| US6159211A (en) * | 1998-10-22 | 2000-12-12 | Depuy Acromed, Inc. | Stackable cage system for corpectomy/vertebrectomy |
| US6126688A (en) * | 1998-12-21 | 2000-10-03 | Surgical Dynamics Inc. | Apparatus for fusion of adjacent bone structures |
| DE29901611U1 (de) * | 1999-01-30 | 1999-04-22 | Aesculap Ag & Co Kg | Chirurgisches Instrument zum Einführen von Zwischenwirbelimplantaten |
| US6770096B2 (en) * | 1999-07-01 | 2004-08-03 | Spinevision S.A. | Interbody spinal stabilization cage and spinal stabilization method |
| US6447543B1 (en) * | 1999-09-28 | 2002-09-10 | Sulzer Orthopedics Ltd. | Basket-like container for implanting bone tissue |
| US6527773B1 (en) * | 1999-10-07 | 2003-03-04 | Osteotech, Inc. | Cervical dowel and insertion tool |
| US6830570B1 (en) * | 1999-10-21 | 2004-12-14 | Sdgi Holdings, Inc. | Devices and techniques for a posterior lateral disc space approach |
| WO2001028469A2 (en) * | 1999-10-21 | 2001-04-26 | Sdgi Holdings, Inc. | Devices and techniques for a posterior lateral disc space approach |
| US6461359B1 (en) * | 1999-11-10 | 2002-10-08 | Clifford Tribus | Spine stabilization device |
| US6319257B1 (en) * | 1999-12-20 | 2001-11-20 | Kinamed, Inc. | Inserter assembly |
| US6648915B2 (en) * | 1999-12-23 | 2003-11-18 | John A. Sazy | Intervertebral cage and method of use |
| DE10011678B4 (de) * | 2000-03-10 | 2007-07-26 | Richard Wolf Gmbh | Chirurgisches Instrument zum Applizieren von Implantaten |
| US6296665B1 (en) * | 2000-03-20 | 2001-10-02 | Electro-Biology, Inc. | Method and apparatus for spinal fixation |
| WO2001085033A2 (en) * | 2000-05-05 | 2001-11-15 | Osteotech, Inc. | Intervertebral distractor and implant insertion instrument |
| US6579318B2 (en) * | 2000-06-12 | 2003-06-17 | Ortho Development Corporation | Intervertebral spacer |
| FR2811543B1 (fr) * | 2000-07-12 | 2003-07-04 | Spine Next Sa | Implant intersomatique |
| KR100371308B1 (ko) | 2000-08-28 | 2003-02-07 | 구자교 | 척추체간 유합용 보철 삽입물 및 그 삽입장치 |
| US6666866B2 (en) * | 2000-11-07 | 2003-12-23 | Osteotech, Inc. | Spinal intervertebral implant insertion tool |
| WO2002058600A2 (en) * | 2001-01-26 | 2002-08-01 | Osteotech, Inc. | Implant insertion tool |
| ATE326925T1 (de) * | 2001-01-30 | 2006-06-15 | Synthes Ag | Knochenimplantat, insbesondere zwischenwirbelimplantat |
| EP1236451B1 (en) * | 2001-02-27 | 2009-04-29 | coLigne AG | Medical implant |
| CA2443001A1 (en) | 2001-04-04 | 2002-10-17 | Lawrence G. Rapp | Interbody spinal fusion device |
| US20030149438A1 (en) * | 2001-04-30 | 2003-08-07 | Howmedica Osteonics Corp. | Insertion instrument |
| US6846327B2 (en) * | 2001-05-01 | 2005-01-25 | Amedica Corporation | Radiolucent bone graft |
| US20050177238A1 (en) * | 2001-05-01 | 2005-08-11 | Khandkar Ashok C. | Radiolucent bone graft |
| US6974480B2 (en) * | 2001-05-03 | 2005-12-13 | Synthes (Usa) | Intervertebral implant for transforaminal posterior lumbar interbody fusion procedure |
| US6719794B2 (en) * | 2001-05-03 | 2004-04-13 | Synthes (U.S.A.) | Intervertebral implant for transforaminal posterior lumbar interbody fusion procedure |
| US20030014115A1 (en) * | 2001-07-16 | 2003-01-16 | Ralph James D. | Insertion tool for use with intervertebral spacers |
| US6428544B1 (en) * | 2001-07-16 | 2002-08-06 | Third Millennium Engineering, Llc | Insertion tool for use with trial intervertebral distraction spacers |
| US6478801B1 (en) * | 2001-07-16 | 2002-11-12 | Third Millennium Engineering, Llc | Insertion tool for use with tapered trial intervertebral distraction spacers |
| US6585749B2 (en) * | 2001-08-01 | 2003-07-01 | Sulzer Spine-Tech Inc. | Surgical implant instrument and method |
| ATE398431T1 (de) * | 2001-08-24 | 2008-07-15 | Zimmer Gmbh | Künstliche bandscheibe |
| DE50114037D1 (de) * | 2001-08-24 | 2008-07-31 | Zimmer Gmbh | Künstliche Bandscheibe |
| EP1429693B1 (en) * | 2001-09-27 | 2006-01-04 | Zimmer Spine, Inc. | Modular spinal fusion device and manufacturing method therefor |
| AU2002340306A1 (en) * | 2001-10-30 | 2003-05-12 | Osteotech, Inc. | Bone implant and insertion tools |
| US20030139812A1 (en) * | 2001-11-09 | 2003-07-24 | Javier Garcia | Spinal implant |
| US6979353B2 (en) * | 2001-12-03 | 2005-12-27 | Howmedica Osteonics Corp. | Apparatus for fusing adjacent bone structures |
| US7238203B2 (en) * | 2001-12-12 | 2007-07-03 | Vita Special Purpose Corporation | Bioactive spinal implants and method of manufacture thereof |
| DK175391B1 (da) | 2002-02-13 | 2004-09-20 | Danfoss As | Implantat til columna og fremgangsmåde til fremstilling heraf |
| US6726720B2 (en) * | 2002-03-27 | 2004-04-27 | Depuy Spine, Inc. | Modular disc prosthesis |
| US7572276B2 (en) * | 2002-05-06 | 2009-08-11 | Warsaw Orthopedic, Inc. | Minimally invasive instruments and methods for inserting implants |
| US7674297B2 (en) * | 2002-06-14 | 2010-03-09 | U.S. Spinal Technologies, Llc | Anatomic vertebral cage |
| EP1542626B1 (en) * | 2002-08-15 | 2012-09-26 | Synthes GmbH | Controlled artificial intervertebral disc implant |
| JP2006516199A (ja) * | 2002-09-24 | 2006-06-29 | ボゴミール ゴレンセク | 椎間円板用の安定化装置および方法 |
| US6712852B1 (en) * | 2002-09-30 | 2004-03-30 | Depuy Spine, Inc. | Laminoplasty cage |
| US6899735B2 (en) * | 2002-10-02 | 2005-05-31 | Sdgi Holdings, Inc. | Modular intervertebral prosthesis system |
| US7232463B2 (en) * | 2002-10-23 | 2007-06-19 | U.S. Spinal Technologies, Llc | Intervertebral cage designs |
| US7192447B2 (en) * | 2002-12-19 | 2007-03-20 | Synthes (Usa) | Intervertebral implant |
| AU2004211984A1 (en) | 2003-02-12 | 2004-08-26 | Warsaw Orthopedic, Inc. | Device for fusing two bone segments |
| US7806932B2 (en) * | 2003-08-01 | 2010-10-05 | Zimmer Spine, Inc. | Spinal implant |
| US7235082B2 (en) * | 2003-08-12 | 2007-06-26 | Depuy Spine, Inc. | Device for insertion of implants |
| US7776093B2 (en) * | 2003-10-20 | 2010-08-17 | Blackstone Medical, Inc. | Vertebral body replacement apparatus and method |
| FR2861582B1 (fr) | 2003-10-29 | 2006-02-10 | Eurosurgical | Cage intersomatique pour fusion lombaire par abord transforaminal et son dispositif porte cage |
| US7137997B2 (en) * | 2003-12-29 | 2006-11-21 | Globus Medical, Inc. | Spinal fusion implant |
| US7959675B2 (en) * | 2005-04-08 | 2011-06-14 | G&L Consulting, Llc | Spine implant insertion device and method |
| US7575580B2 (en) * | 2005-04-15 | 2009-08-18 | Warsaw Orthopedic, Inc. | Instruments, implants and methods for positioning implants into a spinal disc space |
| AU2006292074B2 (en) | 2005-09-26 | 2011-11-24 | Warsaw Orthopedic, Inc. | Hybrid intervertebral spinal fusion implant |
| US20070173820A1 (en) * | 2006-01-13 | 2007-07-26 | Sdgi Holdings, Inc. | Materials, devices, and methods for treating multiple spinal regions including the anterior region |
-
2006
- 2006-09-26 JP JP2008532499A patent/JP2009509590A/ja active Pending
- 2006-09-26 CN CNA2006800352540A patent/CN101272749A/zh active Pending
- 2006-09-26 CN CNA200680035256XA patent/CN101296669A/zh active Pending
- 2006-09-26 CN CNA2006800352555A patent/CN101272750A/zh active Pending
- 2006-09-26 WO PCT/US2006/037549 patent/WO2007038545A1/en active Application Filing
- 2006-09-26 US US11/527,121 patent/US7998212B2/en active Active
- 2006-09-26 EP EP06815501A patent/EP1951164A1/en not_active Withdrawn
-
2011
- 2011-01-14 US US13/006,724 patent/US8728166B2/en active Active
-
2014
- 2014-05-21 US US14/283,613 patent/US20140257492A1/en not_active Abandoned
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105997310A (zh) * | 2016-05-06 | 2016-10-12 | 北京市春立正达医疗器械股份有限公司 | 骨小梁融合器 |
| CN105997310B (zh) * | 2016-05-06 | 2018-03-23 | 北京市春立正达医疗器械股份有限公司 | 骨小梁融合器 |
| US10660766B2 (en) | 2016-05-06 | 2020-05-26 | Beijing Chunlizhengda Medical Instruments Co., Ltd. | Bone trabecular fusion cage |
Also Published As
| Publication number | Publication date |
|---|---|
| US20140257492A1 (en) | 2014-09-11 |
| WO2007038545A1 (en) | 2007-04-05 |
| JP2009509590A (ja) | 2009-03-12 |
| US7998212B2 (en) | 2011-08-16 |
| EP1951164A1 (en) | 2008-08-06 |
| US20110112643A1 (en) | 2011-05-12 |
| US20070093898A1 (en) | 2007-04-26 |
| US8728166B2 (en) | 2014-05-20 |
| CN101272749A (zh) | 2008-09-24 |
| CN101296669A (zh) | 2008-10-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101272750A (zh) | 混合脊柱融合椎间植入体 | |
| JP5291464B2 (ja) | 複合型の椎体間の脊椎固定インプラント | |
| CN101600404B (zh) | 微创模块化体内融合装置的方法及设备 | |
| EP1942837B1 (en) | Anterior hybrid implant | |
| AU772498B2 (en) | Spinal fusion implant | |
| ES2596520T3 (es) | Jaula degradable diseñada revestida con capas minerales para fusión intercorporal espinal | |
| EP1290985B1 (en) | Intersomatic cage for posterior fusion surgery to the lumbar column | |
| US7135042B2 (en) | Surgical implant | |
| US11234832B2 (en) | Support element for implantation into or between subject's bones, and implant component and implant system containing the same | |
| CN202051852U (zh) | 高仿真定制化组合式人工脊椎 | |
| CN102166140A (zh) | 高仿真定制化组合式人工脊椎 | |
| JP2022105749A (ja) | 対角挿入軸を有するインプラント | |
| CN210784858U (zh) | 一种组织工程颈椎椎间融合器 | |
| US20200054455A1 (en) | Interbody standalone device with integrated fixations | |
| CN110169847A (zh) | 一种组织工程颈椎椎间融合器及使用方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |
Open date: 20080924 |